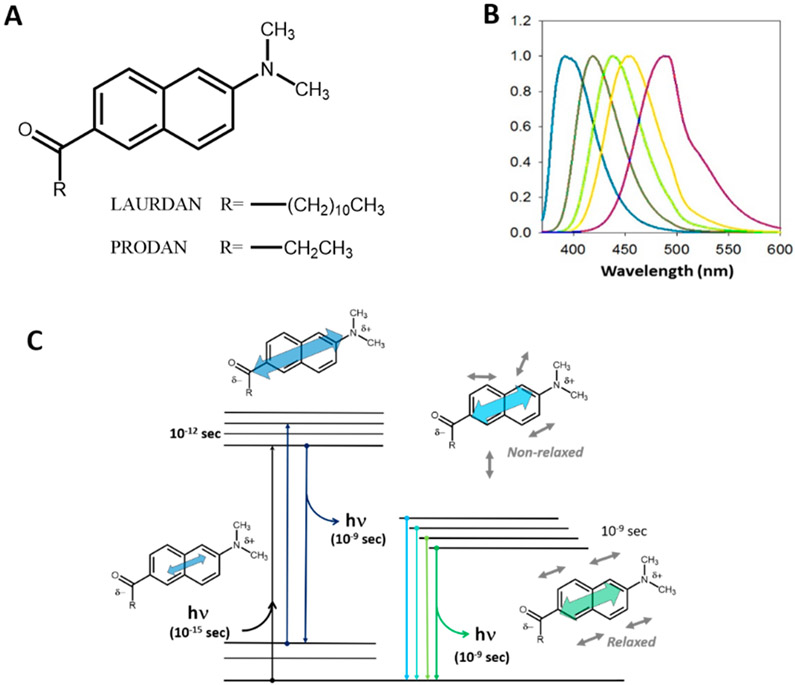
Figure 1.
(A) Chemical structure of LAURDAN and PRODAN. (B) Emission spectra of LAURDAN in different solvents with increasing polarities (from left to right: hexane, benzene, chloroform, acetonitrile, and ethanol). (C) Perrin–Jabłoński diagram for 6-alkyl-2-dimethyl amino naphthalene, showing the ground state and the first singlet excited state. Excitation results in an increase in charge separation and hence an increase in the magnitude of the fluorophore’s dipole moment, which then induces relaxation of the solvent dipoles.