Fig. 7-.
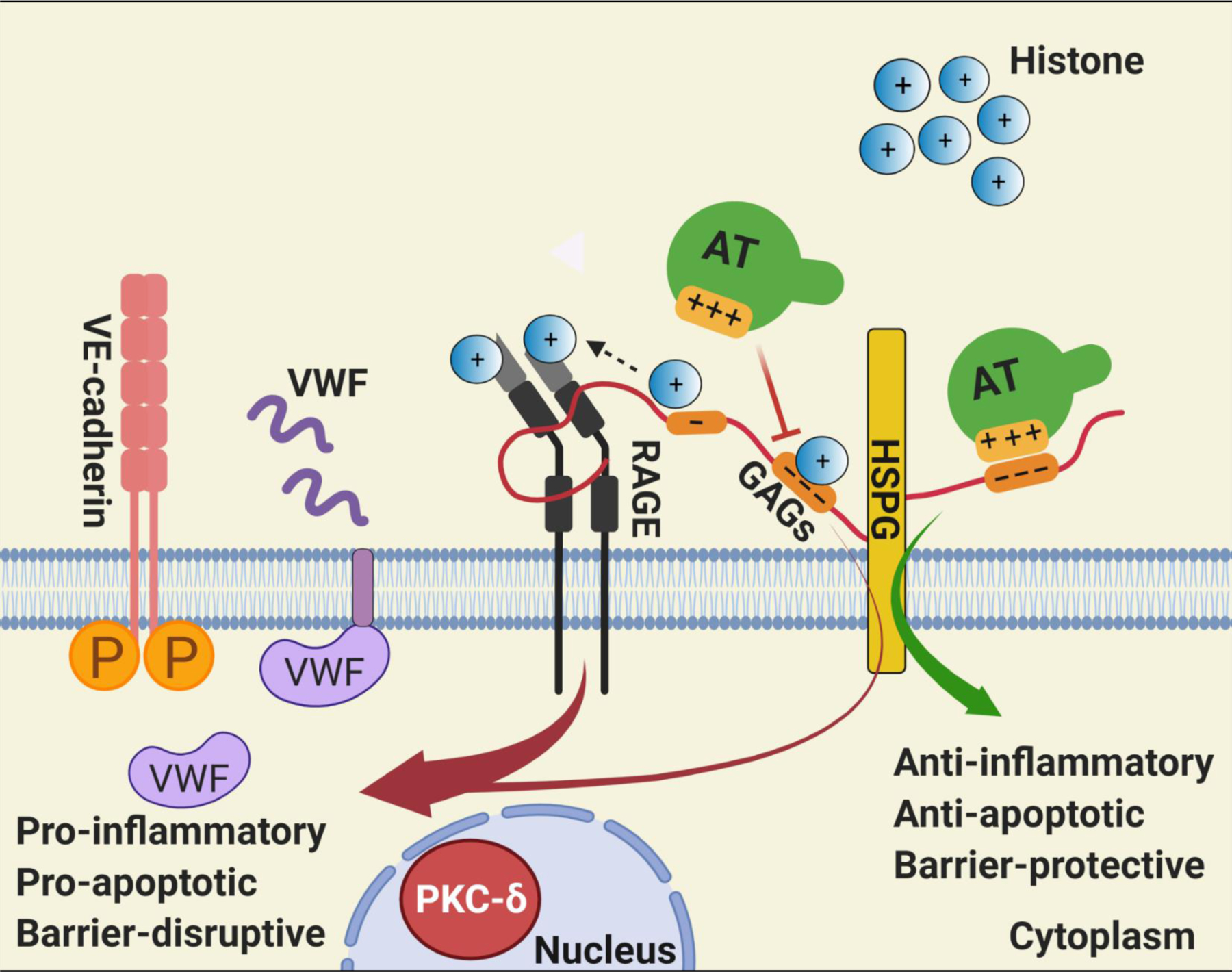
Hypothetical model of GAG-dependent AT and histone signaling in endothelial cells. Binding of positively charged residues of D-helix of AT on distinct GAGs covalently linked to heparan sulfate proteoglycans (HSPG) culminates in anti-inflammatory, anti-apoptotic and barrier-protective effects. Histones can bind to the same and/or overlapping AT-binding sites on GAGs, thereby excluding AT from binding GAGs. GAGs are known to bind RAGE, activate the receptor by oligomerization and also present histones to RAGE [29], thereby promoting the pro-inflammatory, pro-apoptotic and barrier-disruptive signaling functions of nuclear proteins. The GAG-dependent RAGE signaling by histones induces VWF release from endothelial cells. The pro-apoptotic activity of histones is associated with RAGE-dependent nuclear localization of PKC-δ in endothelial cells. The GAG-dependent histone signaling through RAGE also leads to phosphorylation of VE-cadherin and destabilization of endothelial cell junctions. See the text for further details. Figure was prepared by software provided by Biorender.com.
