FIGURE 7.
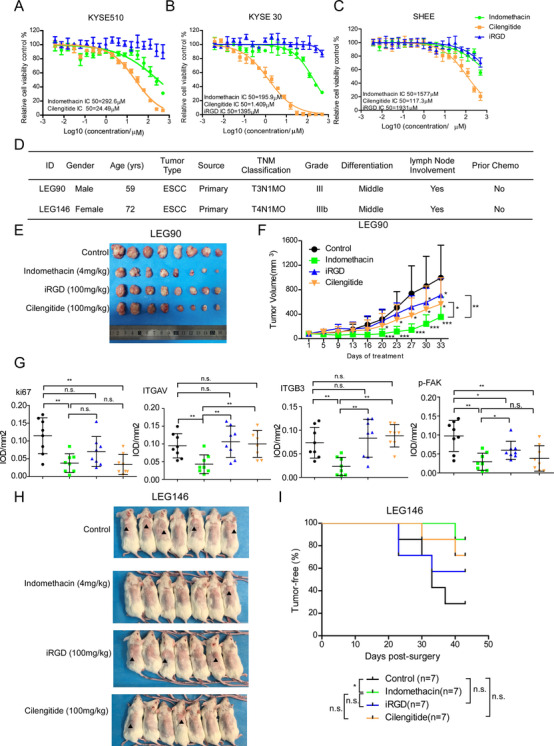
Indomethacin exerts stronger anti‐tumour activities than iRGD and cilengitide in vivo. (A–C) An MTT assay was performed 48 h after treating KYSE30, KYSE510 and SHEE cells with different concentrations of indomethacin, cilengitide and iRGD ranging between 0 and 500 µM for 48 h. (D) ESCC patient information. (E and F) PDX tumour size was plotted over 33 days (LEG90, n = 8/group) to assess the effect of indomethacin, iRGD and cilengitide. Vehicle or indomethacin (4 mg/kg) was administered by intra‐gastric administration. iRGD (100 mg/kg) and cilengitide (100 mg/kg) were administered by IP injection. Tumour volumes were measured twice per week. (G) IHC staining of Ki67, ITGAV, ITGB3, p‐FAK, p‐PI3K, p‐AKT and p‐GSK3β in case LEG90. (H). PDX tumours (case LEG 146) were inoculated into NOD‐SCID mice. After tumour volumes reached approximately 300 mm3, tumours were removed. Mice were randomized and then treated with indomethacin, iRGD or cilengitide for 43 days. Image shows a macroscopic view of mice harbouring recurrent tumours or tumour‐free animals at the end of the experiment. (I) Kaplan–Meier curve depicting recurrence‐free survival in mice treated with the indicated drugs or vehicle control. Recurrence was defined as the presence of a palpable tumour
