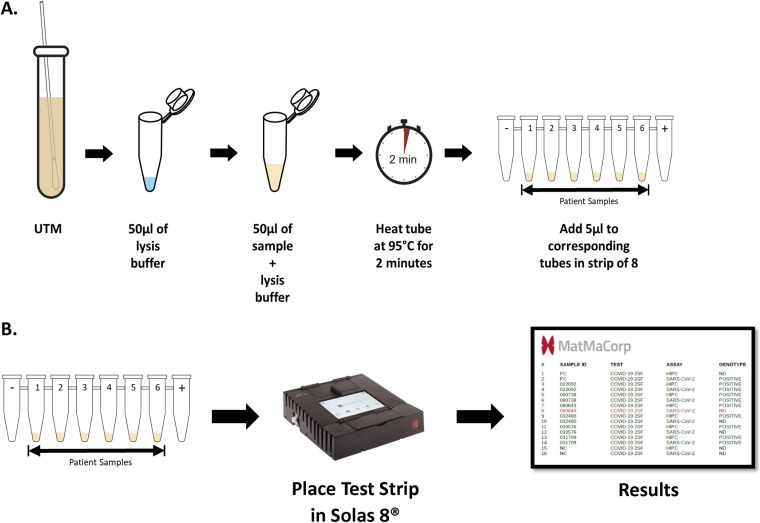
FIG 1.
MatMaCorp COVID-19 2SF test workflow. (A) Equal volumes of nasopharyngeal (NP) swab specimen in universal/viral transport medium (UTM/VTM) are combined with CNL (lysis) buffer in a 1.5-ml lid-locking tube, then heated for 2 min at 95°C on the Solas 8 platform. Only 5 μl of the sample lysate is used in the reaction mixture. The reaction is performed in 8-tube PCR strips. Up to 6 patient samples can be analyzed per run. Tube 1 is a negative control (NC), and tube 8 is a positive control (PC); both are provided in the kit. (B) Reaction tubes are loaded into the Solas 8 instrument for reverse transcription, probe binding, and isothermal amplification. Fluorescent detection is automatically translated into calls of “positive” or “not detected” (ND) for each sample and target. Calls are displayed in real time on the screen of the Solas 8 instrument, and a portable document format (PDF) file with final results is generated at the end of the run.