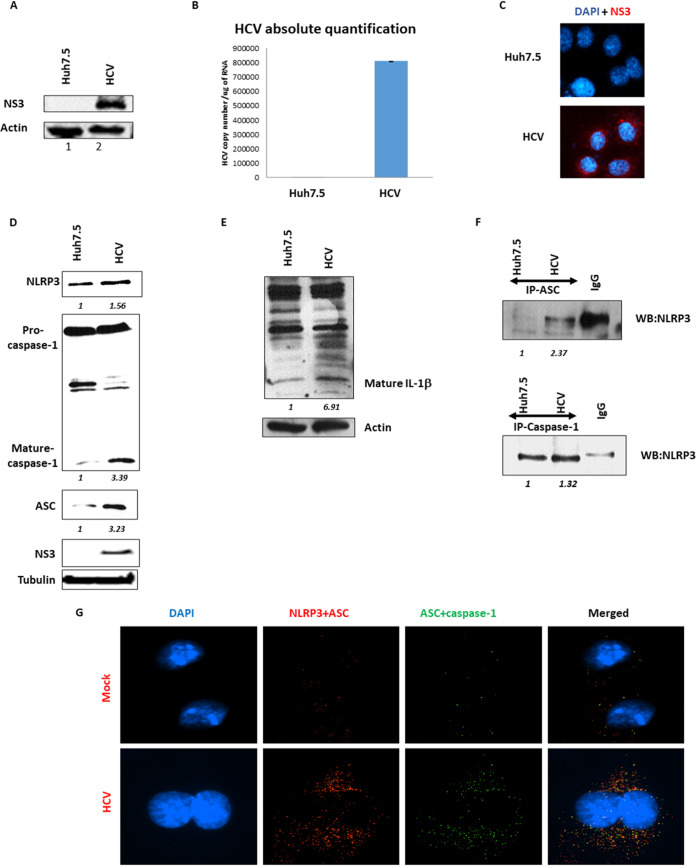
FIG 1.
Hepatitis C virus infection induces NLRP3 inflammasome activation. (A) Huh7.5 (mock) cells were infected with HCV at 1 multiplicity of infection (MOI) and 7 days postinfection (p.i.), and the cells were harvested in RIPA buffer. Western blotting was performed using anti-HCV NS3 and actin antibody. (B) RNA extracted from mock and HCV-infected cells 7 days p.i. was quantified for HCV RNA copy number using RT-PCR. (C) Mock and HCV were fixed using 4% paraformaldehyde and permeabilized using 0.2% Triton X-100. Cells were incubated with anti-HCV NS3 antibody followed by secondary antibody (Red). DAPI is stained in blue. The imaging was performed using a Nikon i80 microscope. (D) Mock and HCV-infected cells were harvested using RIPA lysis buffer and Western blotted for NLRP3, caspase-1, ASC, HCV-NS3, and tubulin. (E) Mock and HCV-infected cells were harvested using RIPA lysis buffer and Western blotted for IL-1β and actin. (F) Mock and HCV-infected cells were harvested using NP-40 lysis buffer, and immunoprecipitation was performed using anti-ASC (top) and anti-caspase-1 (bottom) antibody. Immunoprecipitations were Western blotted for NLRP3 using anti-NLRP3 antibody. (G) Mock and day 7 p.i. HCV-infected cells were permeabilized and fixed as mentioned previously and were subjected to double proximity ligation assay using anti-NLRP3-anti-ASC (red dots) and anti-ASC-anti-caspase-1 (green dots) antibodies. The imaging was performed using a Nikon i80 microscope (scale bars are 10 μm). The merged imaged shows colocalization of the two dots in yellow. Representative images are presented from three independent experiments for panels A to F and two independent experiments for panel G.