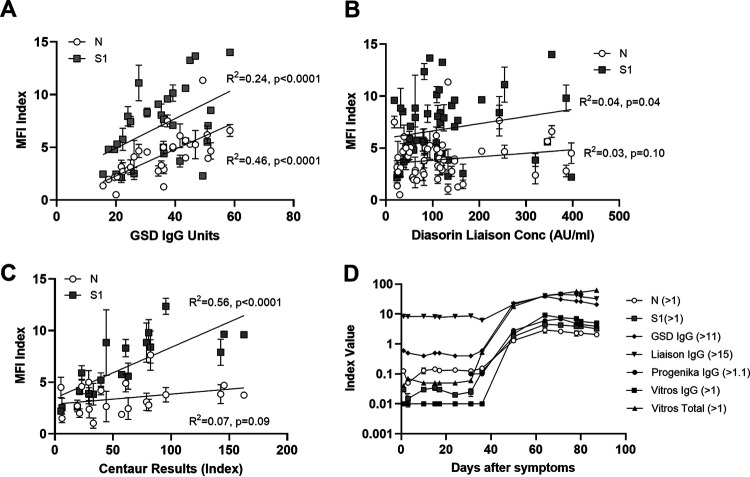
FIG 3.
Concordance with commercially available SARS-CoV-2 immunoassays. Specimen panels from Access Biologicals were tested, and MFI index values for nucleocapsid (N) and spike subunit 1 (S1) were compared to results from other commercial assays as provided by the panel provider. (A) Thirty sera with comparator results from GSD SARS-CoV-2 IgG (Gold Standard Diagnostics). (B) Fifty sera with comparator results from Liaison SARS-CoV-2 S1/S2 IgG (Diasorin). (C) Twenty sera with comparator results from Advia Centaur SARS-CoV-2 total (Siemens). (D) Twenty-eight plasma samples collected from a single individual between days 1 and 98 after COVID-19 symptom onset. The reactive cutoff values for each test are listed next to the name.