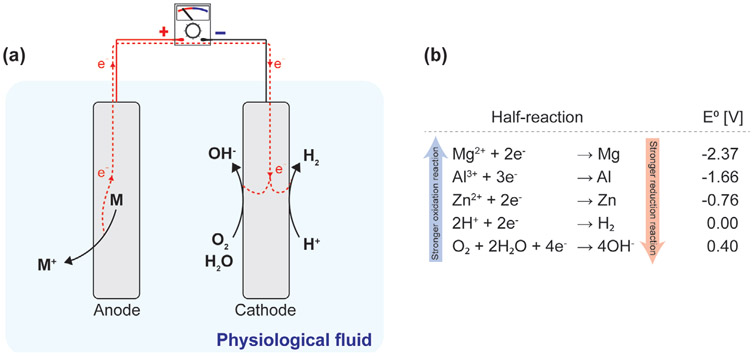
Figure 13.
The working principle of galvanic cells. a) The electrons flow from the oxidation reaction of anode to the reduction reaction of H+ (acidic physiological fluid) or O2 (neutral physiological fluid) at the cathode. b) Standard reduction potential (E0) of typical redox reactions at the anode and cathode.[345]