Scheme 3.
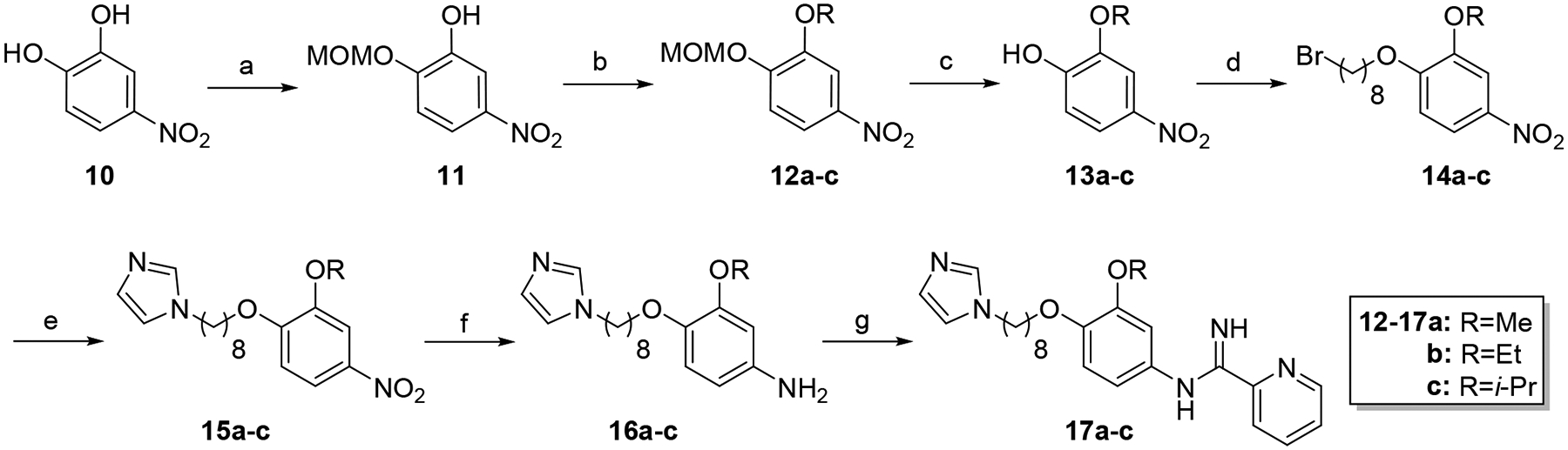
Synthesis of 17a-c. Reagents and conditions: a) MOMCl, K2CO3, DMF, 30°C (48%); b) RI, K2CO3, CH3CN, sealed tube, 80°C (91–97%); c) HCl, MeOH/CH2Cl2, rt (81–90%); d) 1,8-dibromooctane, K2CO3, CH3CN, reflux (84–92%); e) imidazole, K2CO3, CH3CN, reflux (73–90%); f) SnCl2·2H2O, EtOAc, reflux (98–99%); g) naphthalen-2-ylmethyl pyridine-2-carbimidothioate·HBr, CH3CN/EtOH (1:3), rt (31–45%).
