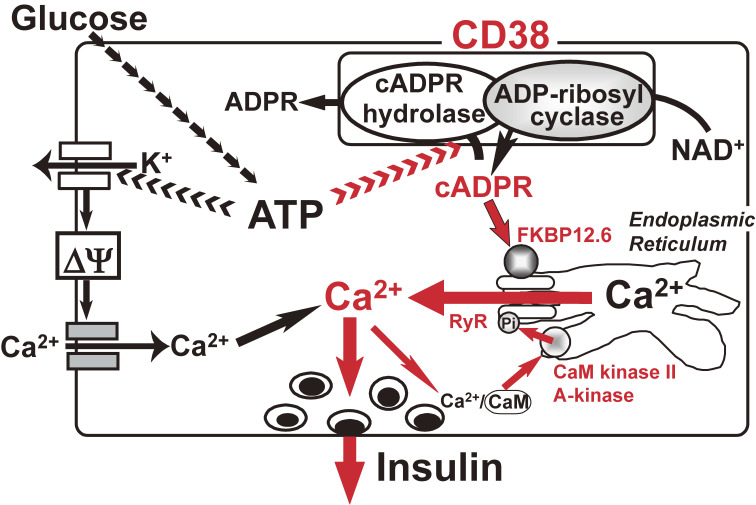
Figure 6.
The CD38-cADPR signal system for insulin secretion by glucose stimulation in β-cells (adapted from Ref. 94). Shown in red is the regulatory pathway for glucose-induced insulin secretion via the CD38-cADPR signal system. ATP competes with cADPR for the binding site (Lys-129, see also Section 5.2), inhibiting the cADPR hydrolysis which causes the accumulation of cADPR. cADPR binds to FKBP12.6 to release Ca2+, dissociating FKBP12.6 from RyR. CaM kinase II phosphorylates RyR to sensitize and activate the Ca2+ channel (Pi, phosphorylation). Ca2+, released from intracellular stores and/or supplied from extracellular sources, further activates CaM kinase II and amplifies the process. In this way, Ca2+-induced Ca2+ release may be explained.288) The conventional insulin secretion mechanism by Ca2+ influx from extracellular sources is shown in black. CaM, calmodulin; RyR, ryanodine receptor; ADPR, ADP-ribose; A-kinase, cyclic AMP-dependent protein kinase.