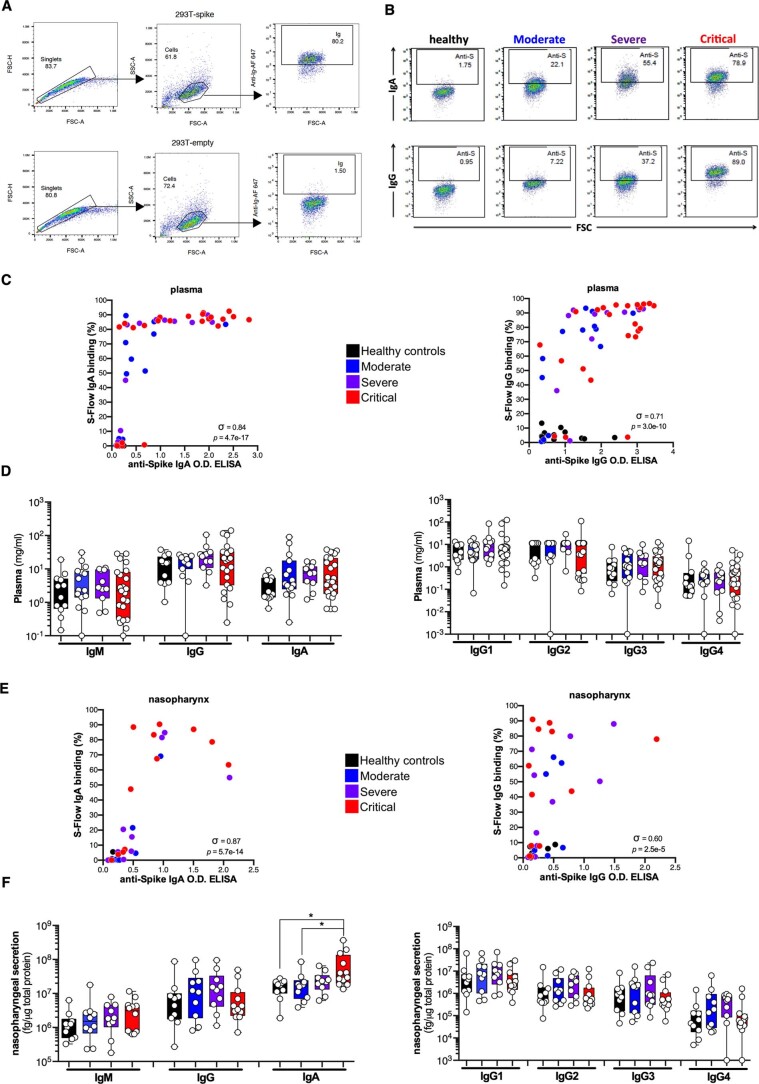
Extended Data Fig. 1. Systemic and mucosal antibody responses in patients with COVID-19.
(a, b) Gating strategy and representative results for the S-Flow assay. (c) Correlation plots between the anti-Spike IgA or IgG OD ELISA and the S-Flow anti-Spike IgA or IgG binding (%) in plasma. (d) Total IgM, IgG and IgA and IgG1/2/3/4 were measured in plasma using a bead-based multiplexed immunoassay system Luminex. (e) Correlation plots between the anti-Spike IgA or IgG OD ELISA and the S-Flow anti-Spike IgA or IgG binding (%) in the nasopharyngeal compartment. (f) Total IgM, IgG and IgA and IgG1/2/3/4 were measured in nasopharyngeal compartment. (a-f) Antibodies were measured in the plasma of healthy controls (n = 12 donors), mild to moderate (n = 15 patients), severe (n = 11 patients) and critical (n = 23 patients) or in the nasopharyngeal compartment of healthy controls (n = 10 donors), mild to moderate (n = 10 patients), severe (n = 10 patients) and critical (n = 12 patients). Nasopharyngeal IgA (Critical vs Healthy, p = 2.9 × 10-2; Critical vs Moderate, p = 4.0 × 10-2). In (c) and (e), σ represents Spearman coefficient and p the p value. In (d) and (f), box-and-whisker plots showing the minimum, maximum, interquartile range and the median. P values were determined with one-sided Kruskal-Wallis test followed by with Dunn’s post-test for multiple group comparisons with Geisser-Greenhouse correction; For all panels: *P < 0.05; **P < 0.01; ***P < 0.001.