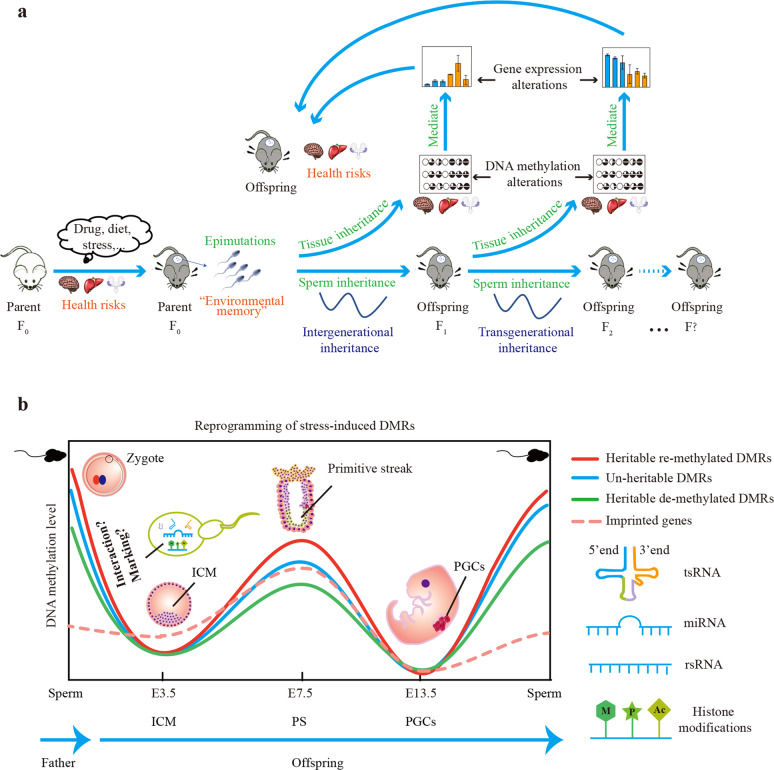
Fig. 7. A model illustrating paternal inheritance of psychological post-stress effects.
a Environmental stimuli, such as long-term psychological stress, could induce health risks in male mice. Simultaneously, a lot of epimutations such as DMRs which represented “epigenetic memory of paternal life experiences” were stored in paternal germ cells. More importantly, notable proportions of these epimutations were epigenetically (including intergenerationally and transgenerationally) inherited by tissues as well as germ cells of the offspring. Subsequently, tissue-inherited epimutations modulated expression patterns of their related genes in relevant tissues, which in turn caused transgenerational transmission of health risks. b Most of the heritable epimutations, including re-methylated and de-methylated, were erased and subsequently reestablished, but not unaltered, during offspring embryonic reprogramming. However, their reestablishment proportions and levels in the PS stage were altered. The heritable re-methylated epimutations had higher methylation reestablishment proportion, while the heritable de-methylated epimutations had lower methylation reestablishment proportion, when compared with the un-heritable epimutations. Meanwhile, most of the heritable de-methylated epimutations had lower reestablishment levels in stress group when compared with control group, whereas most of the heritable re-methylated epimutations had higher reestablishment levels. In addition, the DNA methylation patterns of the heritable epimutations were almost fully cleared in PGCs. Thus, it is likely that some other mechanisms participated in “marking” the heritable status of these heritable epimutations. Histone covalent modifications, such as H3K4me3 and H3K27me3, and sncRNAs, such as miRNAs and tsRNAs, can mediate inheritance of environmental-factors-induced health risks in mammals in a similar manner to DNA methylation. The roles they play in mediating psychological stress-induced paternal inheritance of health risks, through interaction with DNA methylation or marking the heritable status, require further investigation.