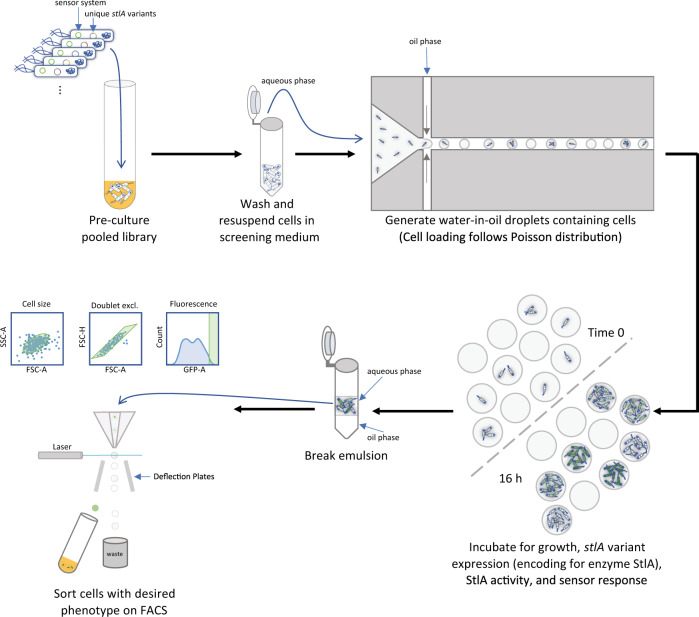
Fig. 2. Depiction of pop ‘n’ sort methodology.
EcN cells harboring the high-copy sensor system plasmid (engineered aTF expression, with aTF-repressed gfp gene encoding for green fluorescent protein GFP) and a library of low-copy inducible stlA variant expression plasmids were grown together within a pool; different cells in this pool contain unique stlA sequences. Following a pre-culture step in a rich medium, the cells are washed and resuspended in an adapted M9 minimal glucose medium for sensor screening, which simultaneously induces stlA expression and provides the substrate Phe. At this stage, the cells are diluted to an OD600 that will result in ~1 cell/droplet loading. These diluted, washed cells in medium serve as the aqueous phase for droplet generation in a fluorinated oil with fluorosurfactant. Because cell loading follows a Poisson distribution, some droplets will be empty and some will contain multiple cells/genotypes at time 0, as shown above. The loaded droplets are incubated until saturation, ~16 h, at which point there are many cells per droplet, and these cells have produced TCA and a per cell GFP signal that is correlated with that TCA production. Since the GFP signal is associated with the cell itself, we can break the emulsions using standard techniques, then sort the cells directly on a FACS for the desired phenotype. See Methods for details on recipes, materials, and protocols.