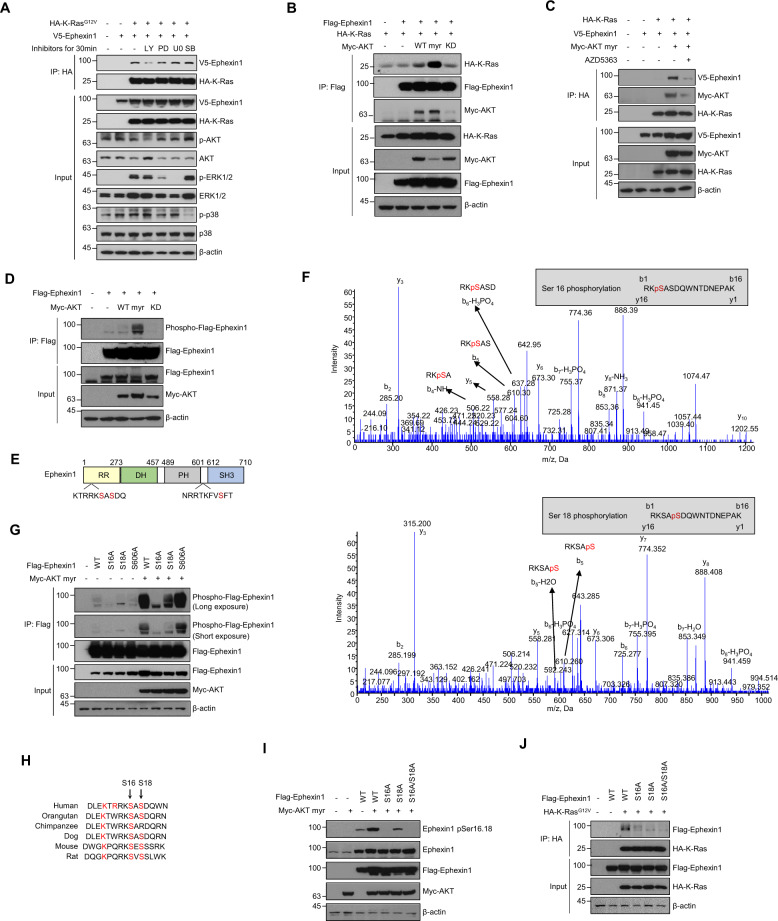
Fig. 4. Akt phosphorylates Ephexin1 at Ser16 and Ser18.
A Immunoprecipitation using an anti-HA antibody and western blot analysis using the indicated antibodies with HEK293T cells transfected with HA-K-RasG12V and V5-Ephexin1, and treated after 48 h with the following inhibitors for 30 min: PI3K inhibitor LY294002 (50 μM), MEK/ERK inhibitors PD98059 (50 μM), and U0126 (10 μM), and p38 inhibitor SB202190 (10 μM). B Co-IP analysis was conducted in HEK293T cells coexpressing Flag-tagged Ephexin1 and HA-tagged K-Ras along with Myc-tagged Akt (WT, myr, or KD). Cell lysates were immunoprecipitated with anti-Flag antibody, and the interaction of Ephexin1 to K-Ras was investigated by western blot using anti-HA antibody. C HEK293T cells were transfected with indicated plasmid, treated with 3 μM AZD5363 for 3 h, and subjected to immunoprecipitation followed by immunoblotting as indicated. D Flag-tagged Ephexin1 was coexpressed with Myc-tagged myr-Akt (WT, myr, or KD) in HEK293T cells. Cell lysates were immunoprecipitated with anti-Flag antibody, and the phosphorylation of Ephexin1 was analyzed by western blot with anti-phospho-Akt substrate antibody. E Schematic diagrams of the Ephexin1 protein domains with putative Akt phosphorylation sites indicated. F Determination of Akt catalyzed phosphorylation sites in Ephexin1 by mass spectrometry. Peptides contain Ser16 and Ser18 phosphorylation are shown at the top and bottom, respectively. G Flag-tagged WT or mutant Ephexin1 was coexpressed with Myc-tagged myr-Akt in HEK293T cells. Cell lysates were immunoprecipitated with an anti-Flag antibody, and the phosphorylation of Ephexin1 was analyzed by western blot with an anti-phospho-Akt substrate antibody. H Amino acid sequence alignment of a region of Ephexin1 with select conserved residues, including Ser16 and Ser18, is indicated in red. I Flag-tagged WT or mutant Ephexin1 was coexpressed with Myc-tagged myr-Akt in HEK293T cells. Phosphorylation of Ephexin1 at Ser16 and Ser18 was analyzed by western blot with anti-pSer16/18 Ephexin1 antibody. J Co-IP analysis was conducted in HEK293T cells cotransfected with HA-tagged K-RasG12V along with Flag-tagged WT or mutant Ephexin1. Cell lysates were immunoprecipitated with anti-HA antibody, and the interaction of K-RasG12V to Ephexin1 mutants was investigated by western blot using anti-Flag antibody.