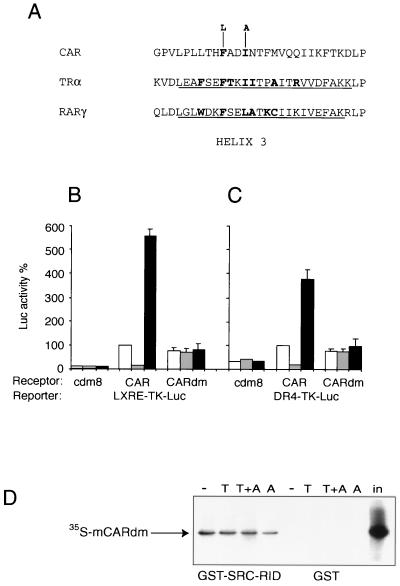
FIG. 7.
Mutations in the ligand binding pocket of CAR block effects of CAR ligands. (A) Mutations in potential ligand contact residues of CAR. The sequence of the putative helix 3 of CAR is aligned to those reported for TRα (30) and RARγ (24). Helical residues in the TR and RAR crystal structures are underlined, and residues that make direct contacts with ligands are in boldface. Hydrophobic residues that project into the binding pocket and make contact with ligands are also present in analogous positions in ERα (4, 26), PR (33), PPARγ (21), and PPARδ (36). (B and C) An expression plasmid carrying the CAR double mutant (CARdm) was transfected into HepG2 cells with the LXRE-TK-Luc (B) or the DR4-TK-Luc (C) as the reporter. In both experiments, the double mutant retained approximately 75% of wild-type activity (white bars) and was resistant to both androstanol (gray bars) and TCPOBOP (black bars). (D) CARdm interaction with SRC-1 in vitro. 35S-labeled CARdm was incubated with beads carrying a GST–SRC-1 RID fusion protein or GST alone in the presence of solvent (DMSO) (−), TCPOBOP (T), or androstanol (A), as indicated. Specifically bound proteins were eluted and resolved by SDS-PAGE. The amount of 35S-labeled CARdm corresponding to 20% of the total input (in) is indicated.