Figure 4.
Deletion of IDRs results in aggregate-like Me31B condensates in vitro
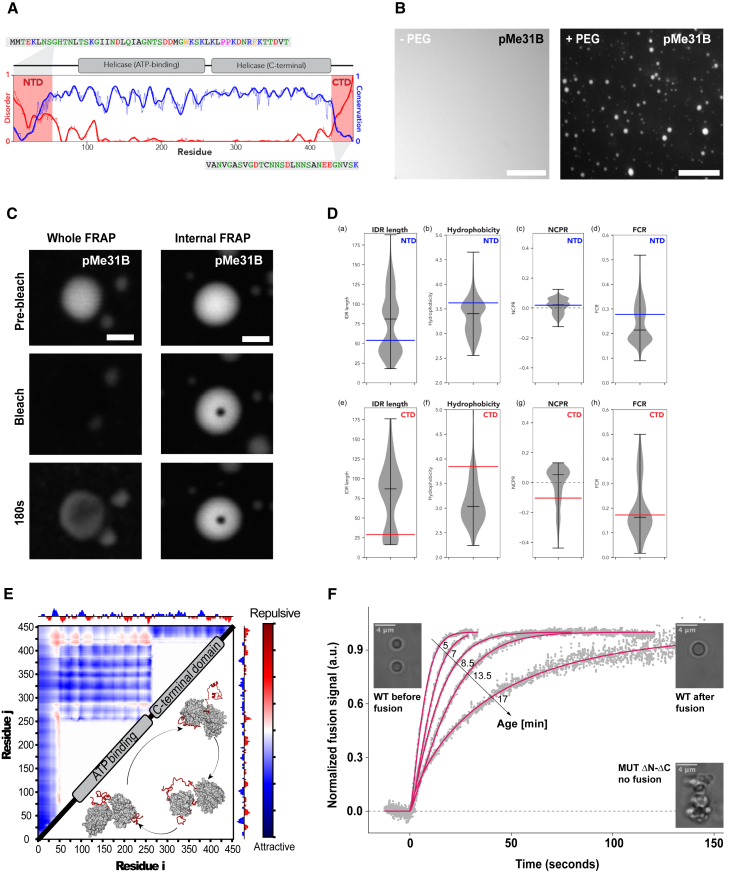
(A) Overview of disordered, conservation, and domain architecture for Me31B. Conservation calculated across 566 orthologous sequences. N-terminal domain (NTD) and C-terminal domain (CTD), sequences are highlighted, with an atomistic model of the full-length protein shown in panel E.
(B) Purified GFP-Me31B (pMe31B) at 7.5 μM is diffuse on its own but forms phase separated spherical condensates in the presence of 1% PEG (n = 10). Maximum projection 5 μm.
(C) Time series of pMe31B condensates subjected to FRAP experiments. Whole P body photobleaching shows moderate fluorescence recovery, whereas internal FRAP shows no recovery (n = 10).
(D) Violin plots quantify density of IDR length (A/E), hydrophobicity (B/F), net charge per residue, (C/G) and fraction of charged residues (D/H) for the N-terminal IDRs (A–D) or C-terminal IDRs (E-H). Blue or red bars define the associated value for the Me31B IDR in the N- or C-terminal IDR, respectively.
(E) Summary of all-atom simulations. Normalized inter-residue distance is shown with cooler colors reflecting attractive interactions and warmer colors reflecting repulsive interactions.
(F) Fusion of pMe31B condensates (magenta) at different time points post condensation, quantified by dual-TRAP optical tweezers. pMe31B ΔN-ΔC condensates (dashed line) do not fuse and rapidly aggregate with each other (n = 20).
Scale bar, 5 μm (B), 1 μm (C).
See also Figure S4.