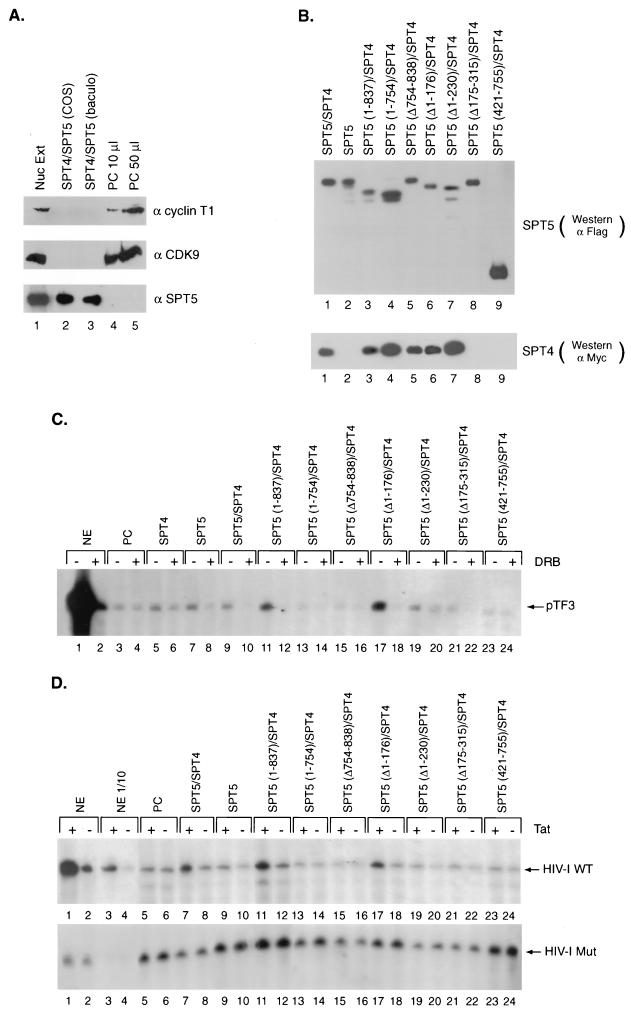
FIG. 4.
Reconstitution of DRB inhibition and Tat activation by recombinant SPT5 proteins. (A) Western blot analysis was performed with antibodies directed against cyclin T1, CDK9, and SPT5 using unfractionated HeLa nuclear extract (Nuc Ext) (lane 1), affinity-purified SPT4-SPT5 complex produced following transfection of epitope-tagged SPT4 and SPT5 cDNAs into COS cells (lane 2), baculovirus-expressed and purified SPT4-SPT5 (lane 3), or 10-μl (lane 4) or 50-μl (lane 5) aliquots of a 1.0 M potassium acetate fraction of HeLa nuclear extract obtained following phosphocellulose (PC) chromatography. (B) Western blot analysis of affinity-purified SPT4 and SPT5 proteins. Expression vectors containing the different Flag-tagged SPT5 cDNA constructs and a Myc-tagged SPT4 cDNA construct were cotransfected into COS cells. The Flag epitope-tagged SPT5 and associated SPT4 protein in these extracts were purified by binding to protein G beads containing M2 monoclonal antibody followed by elution with Flag peptide. Western blot analysis was performed with anti-Flag monoclonal antibody to detect SPT5 proteins (top panel) or anti-Myc monoclonal antibody to detect the associated SPT4 (lower panel). (C) In vitro transcription analysis was performed with the pTF3-6C2AT template using unfractionated HeLa nuclear extract (NE) (lanes 1 and 2), or the 1.0 M potassium acetate fraction of HeLa nuclear extract eluted from the phosphocellulose (PC) column (lanes 3 to 24). The 1.0 M potassium acetate fraction was assayed either alone (lanes 3 and 4), with affinity-purified SPT4 added alone (lanes 5 and 6), with affinity-purified SPT5 added alone (lanes 7 and 8), or with both SPT4 and the different affinity-purified SPT5 proteins shown in panel B added as indicated (lanes 9 to 24). DRB was added to the even-numbered lanes in the in vitro transcription assays. (D) In vitro transcription analysis was performed with the HIV-1 LTR wild-type (top panel) or the HIV-1 LTR loop mutant (lower panel) templates using unfractionated HeLa nuclear extract (lanes 1 to 4), the 1.0 M potassium acetate fraction of HeLa nuclear extract eluted from the phosphocellulose (PC) column alone (lanes 5 and 6), or the 1.0 M potassium acetate fraction in the presence of affinity-purified SPT4 and SPT5 proteins (lanes 7 and 8), SPT5 alone (lane 9 and 10), or SPT4 and affinity-purified SPT5 mutants (lanes 11 to 24) as indicated. Tat (25 ng) was added to the odd-numbered lanes and GST (25 ng) was added to the even-numbered lanes in the in vitro transcription assays. Following the in vitro transcription analysis with these templates containing G-less cassettes, the labeled RNAs were digested with RNase T1 and gel electrophoresis and autoradiography were performed.