Abstract
BACKGROUND:
Communities and individuals widely vary in their resources and ability to respond to external stressors and insults. To identify vulnerable communities, the Centers for Disease Control and Prevention developed the Social Vulnerability Index, an integrated tool to assess community resources and preparedness; it is based on 15 factors and includes individual scores in the following 4 themes: socioeconomic status (theme 1), household composition and disability (theme 2), minority status and language (theme 3), and housing type and transportation (theme 4) and an overall composite score. Several Social Vulnerability Index components have been independently associated with an increased risk of preterm birth.
OBJECTIVE:
We sought to investigate the association of the Social Vulnerability Index for each patient’s residence during pregnancy, personal clinical risk factors, and preterm birth.
STUDY DESIGN:
This was a retrospective cohort study of women carrying nonanomalous singleton or twin gestations delivering at a large university health system from April 2014 to January 2020. Women at high risk of spontaneous and medically indicated preterm birth were assigned to a census tract based on their geocoded home address, and a Social Vulnerability Index score was assigned to each individual by linking each patient’s home address at the census tract level. Higher scores indicate greater social vulnerability. The primary outcome was preterm birth at <37 weeks’ gestation; secondary outcomes were preterm birth at <34 and <28 weeks’ gestation and composite major neonatal morbidity before initial hospital discharge (death, intraventricular leukomalacia or intraventricular hemorrhage, necrotizing enterocolitis, or bronchopulmonary dysplasia). Data were analyzed using the chi-square test, t test, and backward stepwise logistic regression. In addition, because race is a social construct, we conducted regression models omitting Black race. For all regression models, independent variables with a P value of <.20 remained in the final models.
RESULTS:
Overall, 15,364 women met the inclusion criteria, of which 18.5%, 6.5%, 2.1% of women delivered at <37, <34, and <28 weeks’ gestation, respectively, and 3.1% of neonates were diagnosed with major composite morbidity. Women delivering before term at <37, <34, and <28 weeks’ gestation were more likely to live in an area with a higher overall Social Vulnerability Index and higher social vulnerability in each Social Vulnerability Index theme. In regression models, the adjusted odds ratio of preterm birth increased with increasing Social Vulnerability Index scores (across all themes and the composite value); these effects were the greatest at the earliest gestational ages (eg, for the composite Social Vulnerability Index: adjusted odds ratio of preterm birth at <37 weeks’ gestation for models, including Black race, 1.32 [95% confidence interval, 1.14–1.53]; adjusted odds ratio at <34 weeks’ gestation, 1.60 [95% confidence interval, 1.27–2.01]; adjusted odds ratio at <28 weeks’ gestation, 2.21 [95% confidence interval, 1.50–3.25]; adjusted odds ratio for composite major neonatal morbidity, 2.30 [95% confidence interval, 1.67–3.17]). Similar trends were seen for each Social Vulnerability Index theme. In addition, an increased adjusted odds ratio of composite major neonatal morbidity was recognized for each Social Vulnerability Index theme. Results were similar when Black race was removed from the models.
CONCLUSION:
The Social Vulnerability Index is a valuable tool that may further identify communities and individuals at the highest risk of preterm birth and may enable clinicians to integrate information regarding the local home environment of their patients to further refine preterm birth risk assessment.
Keywords: preterm birth, social determinants of health, social vulnerability
Introduction
Preterm birth (PTB; <37 0/7 weeks’ gestation) is the leading cause of neonatal morbidity and mortality and a major public health problem worldwide.1 The pathways (eg, cervical insufficiency, preeclampsia) that ultimately result in prematurity are complex and multifactorial.2 Several risk factors (eg, previous PTB, prepregnancy medical conditions), including some social determinants of health, have been demonstrated to contribute to these pathways.3–6 In addition, community factors, such as residence in a low-income neighborhood or in an area with higher environmental exposure to pollutants, may increase the risk of both spontaneous and medically indicated PTBs.3–8 Finally, in addition to delivery gestational age, there are several factors that influence neonatal morbidity. The relationship between neonatal morbidity and social determinants of health remains understudied, with mixed results.9,10
Identification of communities at the highest risk of PTB based on social determinants of health is challenging. It remains difficult to identify which specific social determinants carry the greatest risk of adverse obstetrical outcomes, as several earlier studies have evaluated individual community-level risk factors or composite indices that focus on limited risk components. The Centers for Disease Control and Prevention’s (CDC) Social Vulnerability Index (SVI) is a publicly available, online database tool that integrates 15 different community characteristics and groups them into 4 different themes to identify “at-risk” communities. These themes are as follows: theme 1, socioeconomic status; theme 2, household composition and disability; theme 3, minority status and language; and theme 4, housing type and transportation.11–13 In addition, a composite SVI—a sum of each of the 4 themes—is calculated to provide an estimated “overall vulnerability.” Detailed information regarding the SVI is available online at the CDC website. Although the SVI was initially developed to assess community emergency preparedness to natural and human-made disasters, this tool has recently been applied in other public health research. For example, other investigators have used the tool to identify communities in the greatest need of interventions to reduce teen pregnancy risk, those areas with the highest morbidity and mortality risks in elderly populations, and to evaluate local disparities in emergency medical services.14–16 Although some of these earlier studies have evaluated SVI in quartiles, other studies have found an increase in adverse outcomes with a single unit (0.01) increase in the SVI.14–16 However, it is unknown whether the SVI corresponding to one’s area of residence during pregnancy is associated with PTB.
Therefore, this study aimed to evaluate whether the SVI in one’s area of residence is associated with PTB. We hypothesized that women living in areas with high social vulnerability, as defined by the SVI, would have a higher odds of PTB before 37 0/7, 34 0/7, and 28 0/7 weeks’ gestation and their neonates would have a higher odds of major morbidity.
Materials and Methods
This was a retrospective cohort study of patients who delivered euploid, nonanomalous singleton or twin gestations at the University of North Carolina Hospital System from April 2014 to January 2020. Patients at high risk of either spontaneous or medically indicated PTB were included. Patients were considered to be high risk of PTB if they had (1) a high-risk medical or surgical history associated with an elevated risk of PTB, diabetes mellitus, chronic hypertension, autoimmune disease (eg, systemic lupus erythematosus, antiphospholipid antibody syndrome), major maternal medical problems (sickle cell disease, chronic renal disease, cardiomyopathy), congenital uterine anomaly, previous uterine or cervical surgery or injury (eg, myomectomy, cervical conization procedure, cervical laceration); (2) ≥1 of the following complications in a previous pregnancy: PTB at <37 0/7 weeks’ gestation for any indication, placental abruption, intrauterine fetal demise, intrauterine growth restriction, cervical cerclage placement, or threatened preterm labor requiring hospitalization; or (3) at least 1 of the following diagnoses in the current pregnancy: cervical insufficiency (defined as asymptomatic cervical dilation before 24 0/7 weeks’ gestation), cervical length shortening of <25 mm at 16 0/7 to 23 6/7 weeks’ gestation identified by transvaginal ultra-sound, or cervical cerclage placement. Study participants were identified using International Classification of Diseases (ICD), Ninth Revision codes (ICD-9) and ICD, Tenth Revision (ICD-10) codes, electronic medical record problem lists, and sonographic reports. Patients who did not ultimately deliver at the University of North Carolina, those with a missing street address (eg, only post office box registered) or a street address that was not geocoded, and those with high-order multiple gestations were excluded.
Each patient’s home address at the time of her initial pregnancy encounter within the University of North Carolina system was geocoded, and a census tract was assigned. Geocoded addresses were then linked at the census tract level to the CDC SVI.11–13 The SVI incorporates 15 indicators and groups them into 4 themes; moreover, a sum composite score is provided. Specifically, theme 1, socioeconomic status, includes the area’s poverty level, unemployment, average income, and population without a high school diploma. Theme 2, household composition and disability, considers the percentage of the area’s younger (<17 years old) and older (>65 years old) citizens, those >5 years old with a disability, and single-parent households. Theme 3, minority status and language, estimates the percentage of the population that is “non-White” and that speaks English “less than well.” Theme 4, housing type and transportation, evaluates the area’s number of multiunit structures, mobile homes, crowding, group quarters, and households without a vehicle. The SVI scores are continuous and unitless and range from 0 to 1, and there is no “threshold” of “high vulnerability.” Furthermore, the SVI scores are generated at the census tract level and based on percentile rankings, with higher values indicating greater vulnerability. The SVI is updated every 2 years based on US Census Bureau data releases. For this analysis, the following SVI data were used: 2014 data for women delivering in 2014 or 2015, 2016 data for women delivering in 2016 or 2017, and 2018 data for women delivering in 2018, 2019, or 2020.11–13
Maternal demographic, previous pregnancy, medical and surgical history, antenatal course, and pregnancy outcomes were obtained through electronic medical record data abstraction, including ICD-9 and ICD-10 codes. In addition, data were obtained through free text data mining from electronic medical record problem lists, which are the primary mechanism of obstetrical documentation for our obstetrical service, and obstetrical ultrasound reports. The delivery gestational age of the pregnancy was calculated from the best obstetrical estimate using a combination of sonographic and menstrual data, per the American College of Obstetricians and Gynecologists criteria.17 The complete records of 685 patients and their neonates were manually verified; an additional 156 records were partially reviewed and “spot-checked.”
The primary outcome was PTB at <37 0/7 weeks’ gestation. Secondary outcomes were PTB at <34 0/7 and 28 0/7 weeks’ gestation and composite major neonatal morbidity (bronchopulmonary dysplasia, necrotizing enterocolitis [surgical or nonsurgical], intraventricular hemorrhage [any grade], periventricular leukomalacia, or death before initial hospital discharge). Neonatal diagnoses were based on ICD-9 and ICD-10 coding at the time of hospital discharge following the delivery admission. Neonatal outcomes were assessed at the pregnancy level; thus, for twin gestations, if either neonate met the criteria for composite morbidity, the pregnancy was considered to have met this adverse outcome.
Data were analyzed using the chi-square test, t test, and backward stepwise logistic regression as appropriate. All initial regression models evaluating PTB as an outcome included several factors a priori known to be associated with prematurity, including Black race, tobacco smoking during pregnancy, chronic hypertension, any diagnosis of diabetes mellitus, a shortened midtrimester cervical length (<25 mm before 24 weeks’ gestation by transvaginal cervical length ultrasound), twin gestation, and male fetal sex. All initial models evaluating composite major neonatal morbidity as an outcome included several factors a priori because of known associations with adverse neonatal outcomes: Black race, tobacco smoking during pregnancy, any diagnosis of diabetes mellitus, twin gestation, small for gestational age (<10% for gestational age and sex), neonatal sepsis, and male fetal sex. For the purposes of regression modeling, all aforementioned factors were dichotomized (yes or no), nulliparous women were considered to have no previous PTB, and women without cervical length ultrasound data available were considered to have a normal midtrimester cervical length. In addition, because race is a social construct, we conducted identical logistic regression models as mentioned above but omitting Black race from the initial models. For all regression models, independent variables with P<.20 remained in the final models. Finally, a Kaplan-Meier survival model was created to evaluate the relationship between the overall SVI score and gestational age as a continuous variable. For the survival analysis, patients with an overall SVI score in the upper quartile for this cohort were considered to have “high” overall social vulnerability and were compared with patients living in areas with an overall SVI score of <75th percentile for this cohort. The equality of the survivor functions was evaluated using the log-rank test. This study was approved by the institutional review board at the University of North Carolina at Chapel Hill under a waiver of informed consent. All analyses were conducted using Stata/MP (version 16.1; StataCorp LLC, College Station, TX).
Results
A total of 15,364 patients met the inclusion criteria, of whom 2241 (18.5%) delivered at <37 0/7, 1001 (6.5%) at <34 0/7, and 327 (2.1%) at <28 0/7 weeks’ gestation. Patients who delivered at <37 0/7 weeks’ gestation were more likely to be of self-reported Black race, carry a diagnosis of diabetes mellitus (pregestational or gestational), have chronic hypertension, report smoking tobacco during pregnancy, have a twin gestation, and be diagnosed with a transvaginal cervical length of <25 mm (Table 1). Overall, 470 pregnancies (3.1%) were affected by at least 1 neonate who developed composite major neonatal morbidity (Table 2). Delivery was at a median of 28.4 (interquartile range 22.7–33.5) weeks’ gestation for pregnancies where at least one neonate developed major neonatal morbidity, and as expected, composite major neonatal morbidity occurred most frequently at the earliest delivery gestational ages (≥37 0/7 weeks’ gestation, 102/12,523 [0.8%]; <37 0/7 weeks’ gestation, 368/2473 [13.0%]; <34 0/7 weeks’ gestation, 323/1001 [32.3%]; <28 0/7 weeks’ gestation, 232/327 [71.0%]). Death and intraventricular hemorrhage or periventricular leukomalacia were the most common individual neonatal morbidities (Table 2).
TABLE 1.
Demographic and pregnancy characteristics of the study population
| Characteristic | Overall (N=15,364) | Preterm birth at <37 wk (n=2841) | Term birth at ≥37 wk (n=12,523) | P value | |
|---|---|---|---|---|---|
| Maternal age at due date (y), mean±SD | 31.7±5.6 | 31.5±5.9 | 31.7±5.6 | .046 | |
| Multiparous | 8942 (58.2) | 1684 (59.3) | 7258 (58.0) | .199 | |
| Previous term delivery at ≥37 0/7 wka | 6708 (75.0) | 983 (58.4) | 5725 (78.9) | <.001 | |
| Black race | 3643 (23.7) | 763 (26.9) | 2880 (23.0) | <.001 | |
| Hispanic ethnicity | 2335 (15.2) | 378 (13.3) | 1957 (15.6) | .002 | |
| Smoked during pregnancy | 1029 (6.7) | 257 (9.1) | 772 (6.2) | <.001 | |
| High-risk medical or surgical history | Any diabetes mellitus diagnosis (pregestational or gestational) | 5335 (34.7) | 713 (25.1) | 4622 (36.9) | <.001 |
| Chronic hypertension | 2661 (17.3) | 644 (22.7) | 2017 (16.1) | <.001 | |
| Autoimmune disease (systemic lupus erythematosus, antiphospholipid antibody syndrome, sarcoidosis) | 233 (1.5) | 28 (1.0) | 205 (1.6) | .010 | |
| Other major maternal medical problems (sickle cell disease, chronic renal disease, cardiomyopathy, peripartum cardiomyopathy, congestive heart failure, myocardial infarction) | 122 (0.8) | 25 (0.9) | 97 (0.8) | .568 | |
| Congenital uterine anomaly | 177 (1.4) | 29 (1.2) | 148 (1.4) | .344 | |
| Previous uterine or cervical surgery or injury (eg, myomectomy, cervical conization procedure, cervical laceration) | 529 (3.4) | 95 (3.3) | 434 (3.5) | .748 | |
| High-risk obstetrical history | Previous preterm delivery at 16 0/7 to 36 6/7 wka | 3579 (40.0) | 1003 (59.7) | 2576 (35.5) | <.001 |
| Previous pregnancy complicated by placental abruptiona | 240 (2.7) | 69 (4.1) | 171 (2.4) | <.001 | |
| Previous pregnancy complicated by preeclampsia or eclampsiaa | 1531 (17.1) | 324 (19.2) | 1207 (16.6) | .010 | |
| Previous pregnancy complicated by cervical insufficiency or requiring cervical cerclage placementa | 424 (4.7) | 153 (9.1) | 271 (3.7) | <.001 | |
| History of intrauterine fetal demisea | 330 (3.7) | 76 (4.5) | 254 (3.5) | .047 | |
| Previous pregnancy complicated by small-for-gestational-age neonatea | 76 (0.9) | 13 (0.8) | 63 (0.9) | .699 | |
| History of threatened preterm labor requiring hospitalization (without preterm delivery)a | 939 (10.5) | 270 (16.0) | 669 (9.2) | <.001 | |
| High risk of preterm birth because of conditions present in the current pregnancy | Asymptomatic cervical dilation diagnosed before 24 0/7 wk | 64 (0.4) | 37 (1.3) | 27 (0.2) | <.001 |
| Transvaginal cervical length <25 mm between 16 0/7 and 23 6/7 wk | 282 (1.8) | 113 (4.0) | 169 (1.4) | <.001 | |
| Cervical cerclage placed during pregnancy | 462 (3.0) | 167 (5.9) | 295 (2.4) | <.001 | |
| Twin gestation | 902 (5.9) | 492 (17.4) | 409 (3.3) | <.001 | |
Data are presented as number (percentage), unless otherwise indicated.
SD, standard deviation.
Among 8942 multiparous women.
TABLE 2.
Delivery characteristics and initial neonatal outcomes
| Characteristic | Overall (n=15,364) | Preterm birth at <37 wk (n=2841) | Term birth at ≥37 wk (n=12,523) | P value |
|---|---|---|---|---|
| Delivery gestational age (wk), median (IQR) | 39.0 (37.4–39.5) | 35.2 (33.0–36.4) | 39.1 (38.3–39.7) | <.001 |
| Cesarean delivery | 6061 (39.5) | 1331 (46.9) | 4730 (37.8) | <.001 |
| Birthweight (g), mean±SD | ||||
| Twin gestations | 2431 ±466 | 2256±465 | 2655±360 | <.001 |
| Male fetusa | 7893 (51.4) | 1522 (53.8) | 6371 (50.9) | .005 |
| Neonatal morbidityb | ||||
| Bronchopulmonary dysplasia | 87 (0.6) | 63 (2.2) | 24 (0.20) | <.001 |
| Intraventricular hemorrhage or periventricular leukomalacia | 100 (0.7) | 82 (2.9) | 18 (0.1) | <.001 |
| Necrotizing enterocolitis | 23 (0.2) | 23 (0.8) | 0 (0.0) | <.001 |
| Death | 257 (1.7) | 195 (6.9) | 62 (0.5) | <.001 |
| Composite major neonatal morbidity or mortality (one or more of the aforementioned neonatal morbidities)c | 470 (3.1) | 368 (13.0) | 102 (0.8) | <.001 |
Data are presented as number (percentage), unless otherwise indicated.
IQR, interquartile range; SD, standard deviation.
For twin gestations, fetal sex corresponds to baby A; fetal sex unavailable for 14 subjects;
Before initial hospital discharge following delivery. For twin gestations, all morbidities were evaluated at the level of the pregnancy; thus, if either neonate met the criteria for the listed diagnoses, the pregnancy was considered to have met the adverse outcome;
Some neonates had more than 1 morbidity; thus, the sum of individual morbidities is greater than the composite in each column.
Patients in this cohort resided in 784 different census tracts; the number of patients living in each census tract ranged from 1 to 166 (median, 13; mean, 19.6 patients per census tract). Within the overall cohort, the median overall composite SVI score was 0.45 (IQR, 0.20–0.71), and the scores ranged from 0 to 1.0. Those who delivered at <37 0/7 weeks’ gestation resided in 591 different census tracts, and the number of patients delivering before term in each census tract ranged from 1 to 30 (median, 4; mean, 4.8 patients per census tract). Among those delivering at <37 0/7 weeks’ gestation, the median overall composite SVI score was 0.49 (IQR, 0.21–0.74), and the scores ranged from 0.01 to 1.00.
Individuals with PTB at <37, <34, and <28 weeks’ gestation were more likely to live in an area with a higher overall SVI and higher vulnerability in each of the 4 SVI themes (Table 3). Notably, SVI scores were inversely proportional to gestational age at delivery; the highest SVI scores were noted among those delivering at the earliest gestational ages. Similar findings were appreciated with composite major neonatal morbidity (Table 3).
TABLE 3.
Social Vulnerability Index values, stratified by women with and without preterm births at each gestational age cutoff
| Outcome | SVI component Theme 1: socioeconomic status | Theme 2: household composition and disability | Theme 3: minority status and language | Theme 4: housing type and transportation | Overall composite | |
|---|---|---|---|---|---|---|
| Overall cohort (n=15,364) | 0.41 (0.41–0.42) | 0.43 (0.42–0.43) | 0.60 (0.60–0.61) | 0.50 (0.49–0.50) | 0.46 (0.46–0.47) | |
| Term birth at ≥37 wk (n=12,523) | 0.41 (0.40–0.41) | 0.42 (0.42–0.43) | 0.60 (0.60–0.61) | 0.51 (0.50–0.52) | 0.43 (0.44–0.44) | |
| Preterm birth | <37 0/7 wk (n=2841) | 0.43 (0.42–0.45) P<.001 |
0.45 (0.44–0.46) P<.001 |
0.61 (0.60–0.62) P=.139 |
0.51 (0.50–0.52) P=.002 |
0.49 (0.47–0.50) P<.001 |
| <34 0/7 wk (n=1001) | 0.45 (0.44–0.47) P<.001 |
0.46 (0.44–0.48) P<.001 |
0.63 (0.61–0.64) P=.007 |
0.53 (0.51–0.55) P=.001 |
0.51 (0.49–0.52) P<.001 |
|
| <28 0/7 wk (n=327) | 0.49 (0.46–0.52) P<.001 |
0.48 (0.45–0.52) P<.001 |
0.65 (0.62–0.67) P=.004 |
0.55 (0.51–0.58) P=.002 |
0.54 (0.50–0.57) P<.001 |
|
| No composite major neonatal morbidity (n=14,894) | 0.41 (0.41–0.42) | 0.43 (0.42–0.43) | 0.60 (0.60–0.61) | 0.50 (0.49–0.50) | 0.46 (0.46–0.47) | |
| Composite major neonatal morbidity | All gestational ages (n=470) | 0.48 (0.45–0.51) P<.001 |
0.49 (0.47–0.52) P<.001 |
0.64 (0.62–0.66) P=.002 |
0.55 (0.52–0.57) P<.001 |
0.53 (0.51–0.56) P<.001 |
| <37 0/7 wk (n=368) | 0.47 (0.44–0.50) P<.001 |
0.49 (0.46–0.52) P<.001 |
0.64 (0.61–0.67) P=.005 |
0.54 (0.51–0.57) P<.001 |
0.53 (0.50–0.56) P<.001 |
|
| <34 0/7 wk (n=323) | 0.47 (0.44–0.51) P<.001 |
0.49 (0.46–0.53) P<.001 |
0.64 (0.61–0.67) P=.005 |
0.55 (0.51–0.58) P<.001 |
0.53 (0.50–0.56) P<.001 |
|
| <28 0/7 wk (n=232) | 0.47 (0.43–0.51) P<.001 |
0.48 (0.44–0.52) P<.001 |
0.65 (0.62–0.68) P=.001 |
0.54 (0.50–0.58) P<.001 |
0.53 (0.49–0.57) P<.001 |
|
Data are presented as mean (95% confidence interval). The P values listed are compared with women term births or those without composite major neonatal morbidity, as applicable.
SVI, Social Vulnerability Index.
Individual components of the SVI, stratified by the primary outcome of PTB at <37 0/7 weeks’ vs term delivery, are shown in Table 4. In contrast to the SVI values, the data in Table 4 reflect estimates for each indicator (eg, the percentage of individuals in each census tract below the poverty level). Importantly, each of the 15 individual indicators that compose the SVI was “worse” in the areas where women delivering before term resided, except for the percentage of housing structures with >10 units (3.4% [95% confidence interval (CI), 0.3–14.5] for PTB cases vs 4.1% [95% CI, 0.2%–17.6%] for term controls; P<.001). In a sensitivity analysis excluding patients with twin gestations (n=921), results were similar (data not shown).
TABLE 4.
Individual components of each Social Vulnerability Index theme at the census tract level, reflecting the place of residence of women during pregnancy with and without preterm births at <37 weeks’ gestation
| Theme | SVI componenta | Overall (N=15,264) | Preterm birth at <37 0/7 wk (n=2841) | Term birth at ≥37 0/7 wk (n=12,523) | P value |
|---|---|---|---|---|---|
| Theme 1: socioeconomic status | 12.2 (6.9–20.0) 6.0 (4.0–9.0) 27,575 (21,268–37,708) 10.3 (4.8–17.8) |
12.8 (7.2–20.8) 6.2 (4.2–9.6) 26,814 (20,775–36,130) 11.0 (5.0–19.2) |
12.0 (6.9–19.8) 5.9 (4.0–8.9) 27,727 (21,331–38,052) 10.1 (4.8–17.6) |
<.001 <.001 <.001 <.001 |
|
| Theme 2: household composition, disability |
|
10.9 (7.5–15.0) 12.1 (8.4–15.8) 24.3 (20.7–28.3) 9.2 (6.3–13.3) |
11.2 (7.6–15.2) 12.0 (8.6–15.8) 24.5 (20.8–28.3) 9.5 (6.5–13.6) |
10.8 (7.4–14.9) 12.1 (8.4–15.8) 24.2 (20.6–28.3) 9.1 (6.3–13.2) |
.006 >.99 .056 .009 |
| Theme 3: minority status, language |
|
35.9 (24.2–53.9) 2.1 (0.8–4.7) |
36.7 (24.3–57.1) 2.1 (0.8–5.0) |
35.7 (24.2–53.5) 2.1 (0.8–4.7) |
.012 .358 |
| Theme 4: housing type and transportation |
|
3.9 (0.2–16.3) 4.3 (0.6–15.9) 2.0 (0.7–3.7) 3.7 (1.8–7.3) 0.2 (0.0–1.1) |
3.4 (0.3–14.5) 5.0 (0.6–16.5) 2.2 (0.8–3.9) 4.0 (1.8–7.6) 0.2 (0.0–1.2) |
4.1 (0.2–17.6) 4.0 (0.5–15.7) 2.0 (0.7–3.6) 3.7 (1.8–7.2) 0.2 (0.0–1.1) |
.009 .010 <.001 .031 .035 |
Data are presented as median (interquartile range), unless otherwise indicated.
SVI, Social Vulnerability Index.
Based on percentage values;
Among individuals aged ≥25 years old.
In regression models, for each 0.01 increase in each of the 4 SVI themes and the overall SVI, the adjusted odds ratio (aOR) of PTB increased (Table 5). Consistent with unadjusted analyses, the aORs were the greatest at the earliest gestational age cutoffs. These aORs were the largest for theme 1 (socioeconomic, which includes poverty, unemployment, education) and theme 2 (household composition and disability, which includes single-parent households, individuals aged <17 or >65 years old living in the household, and civilian individuals aged >5 years old with a disability).
TABLE 5.
Unadjusted odds ratio and adjusted odds ratio for preterm births at <37 0/7, <34 0/7, and <28 0/7 weeks’ gestation and composite major neonatal morbidity conferred by each 0.01 increase in the Social Vulnerability Index component
| Unadjusted analyses | Adjusted analyses | ||
|---|---|---|---|
| SVI component | OR (95% CI) | Model A aOR (95% CI) | Model B aOR (95% CI) |
| Theme 1: socioeconomic status | |||
| Preterm birth at <37 0/7 wk | 1.36 (1.19–1.57) | 1.23 (1.06–1.43) | 1.26 (1.08–1.46) |
| Preterm birth at <34 0/7 wk | 1.66 (1.06–1.68) | 1.33 (1.06–1.68) | 1.50 (1.20–1.88) |
| Preterm birth at <28 0/7 wk | 2.47 (1.72–3.54) | 1.97 (1.34–2.90) | 2.35 (1.61–3.42) |
| Composite major neonatal morbidity | 2.18 (1.61–2.96) | 1.85 (1.34–2.56) | 2.22 (1.62–3.05) |
| Theme 2: household composition and disability | |||
| Preterm birth at <37 0/7 wk | 1.33 (1.16–1.53) | 1.28 (1.10–1.48) | 1.31 (1.13–1.52) |
| Preterm birth at <34 0/7 wk | 1.49 (1.20–1.85) | 1.30 (1.03–1.64) | 1.44 (1.15–1.82) |
| Preterm birth at <28 0/7 wk | 1.92 (1.33–2.39) | 1.61 (1.09–2.39) | 1.89 (1.29–2.78) |
| Composite major neonatal morbidity | 2.16 (1.58–2.95) | 1.92 (1.38–2.67) | 2.24 (1.62–3.09) |
| Theme 3: minority status and language | |||
| Preterm birth at <37 0/7 wk | 1.13 (0.96–1.32) | 1.13 (0.95–1.34) | 1.18 (1.00–1.39) |
| Preterm birth at <34 0/7 wk | 1.41 (1.10–1.81) | 1.23 (0.94–1.60) | 1.46 (1.12–1.89) |
| Preterm birth at <28 0/7 wk | 1.90 (1.23–2.93) | 1.47 (0.93–1.33) | 1.88 (1.20–2.85) |
| Composite major neonatal morbidity | 1.80 (1.25–2.59) | 1.44 (0.98–2.13) | 1.86 (1.28–2.71) |
| Theme 4: housing type and transportation | |||
| Preterm birth at <37 0/7 wk | 1.25 (1.09–1.45) | 1.22 (1.04–1.42) | 1.25 (1.07–1.45) |
| Preterm birth at <34 0/7 wk | 1.50 (1.19–1.87) | 1.28 (1.01–1.63) | 1.44 (1.14–1.82) |
| Preterm birth at <28 0/7 wk | 1.82 (1.24–2.68) | 1.40 (0.93–2.10) | 1.64 (1.11–2.44) |
| Composite major neonatal morbidity | 1.87 (1.35–2.59) | 1.47 (1.05–2.07) | 1.74 (1.25–2.43) |
| Overall composite | |||
| Preterm birth at <37 0/7 wk | 1.34 (1.17–1.54) | 1.28 (1.10–1.49) | 1.32 (1.14–1.53) |
| Preterm birth at <34 0/7 wk | 1.39 (1.10–1.75) | 1.47 (1.27–1.71) | 1.60 (1.27–2.01) |
| Preterm birth at <28 0/7 wk | 2.35 (1.62–3.40) | 1.81 (1.21–2.69) | 2.21 (1.50–3.25) |
| Composite major neonatal morbidity | 2.30 (1.68–3.14) | 1.88 (1.35–2.62) | 2.30 (1.67–3.17) |
All initial “A” models evaluating preterm birth as an outcome included Black race, tobacco smoking during pregnancy, chronic hypertension, any diagnosis of diabetes mellitus, a shortened midtrimester cervical length (<25 mm before 24 weeks’ gestation by transvaginal cervical length ultrasound), twin gestation, and male fetal sex. All initial “A” models evaluating composite major neonatal morbidity included Black race, tobacco smoking during pregnancy, any diagnosis of diabetes mellitus, twin gestation, small for gestational age (<10% for gestational age and sex), neonatal sepsis, and male fetal sex. All initial “B” models were identical to “A” models, but patient race was excluded from model inclusion. For all models, factors with P<.20 remained in the final models.
aOR, adjusted odds ratio; CI, confidence interval; SVI, Social Vulnerability Index.
Similarly, the aOR of composite major neonatal morbidity was significantly higher for each individual SVI theme and the overall SVI. As described in the Materials and Methods section, 2 sets of logistic regression models were considered: one that excluded Black race as an independent variable and the other that included Black race. In all models that included Black race, this independent variable remained statistically significant in each model (P<.05). Furthermore, we observed higher aOR for PTB at each gestational age cutoff and a higher aOR of composite major neonatal morbidity when Black race was included in the models (Table 5).
Finally, findings were consistent in the Kaplan-Meier survival analysis evaluating the difference in delivery gestational age. Those living in an area with an overall SVI score of ≥75th percentile for this cohort (0.7075) were considered to have “high” overall social vulnerability and were compared with individuals living in an area with an overall SVI score <75th percentile. Furthermore, we found that those with high overall social vulnerability defined in this fashion were more likely to deliver earlier across a spectrum of gestational ages (log-rank test; P<.001) (Figure).
FIGURE. Kaplan-Meier survival curve.
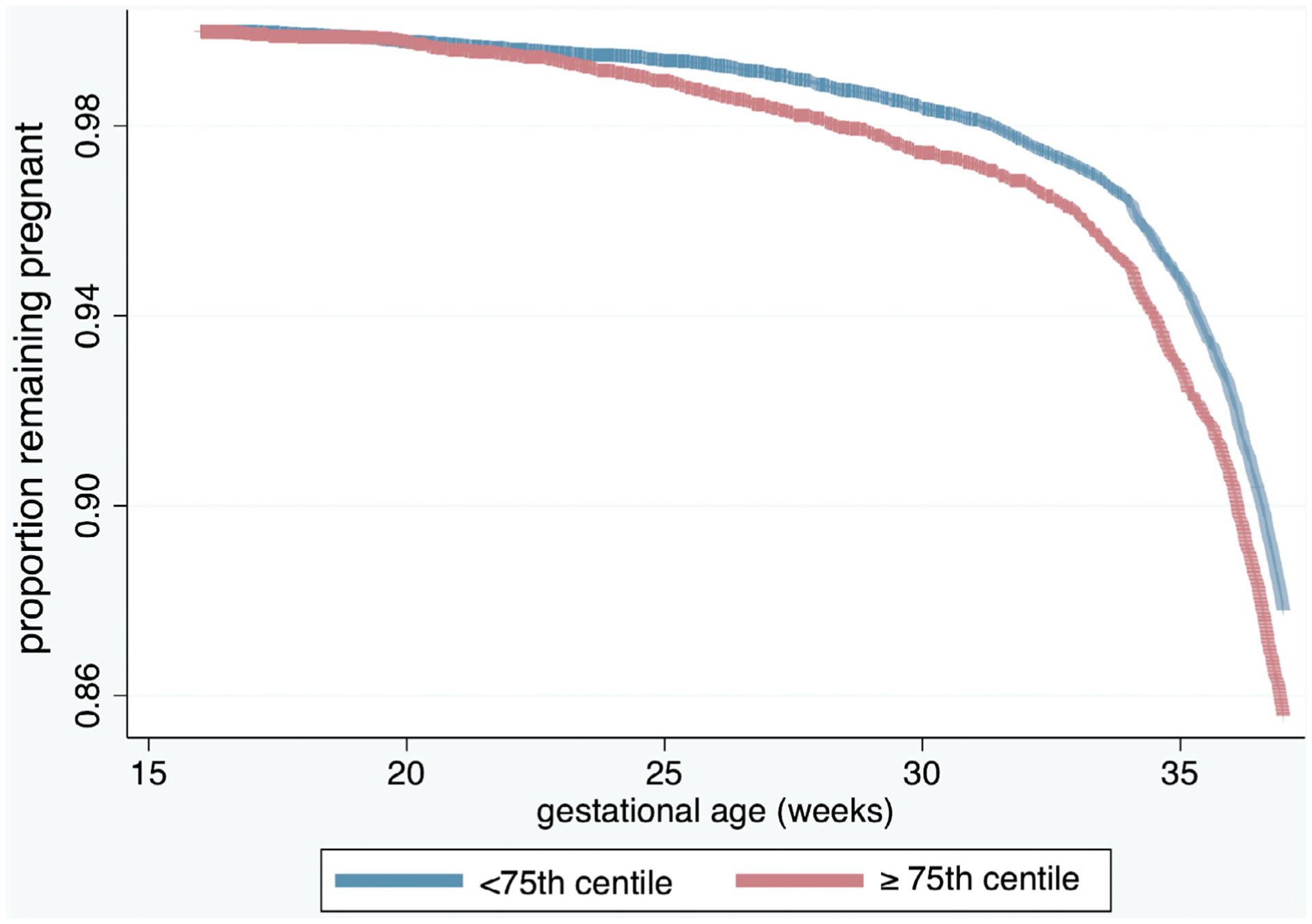
The Kaplan-Meier survival curve (controlling for Black race, tobacco smoking during pregnancy, chronic hypertension, any diagnosis of diabetes mellitus, a shortened midtrimester cervical length [<25 mm before 24 weeks’ gestation by transvaginal cervical length ultrasound], twin gestation, and male fetal sex) demonstrates the probability of remaining pregnant, stratified by patient residence in a neighborhood with an overall Social Vulnerability Index of <75th or ≥75th percentile for this cohort.
Discussion
Principal findings
We found that patients living in census tract-level geographic areas with higher levels of social vulnerability in multiple domains had a higher aOR of PTB at each gestational age cutoff, and their neonates had an increased aOR of composite major neonatal morbidity. All 4 themes composed of the SVI were independently associated with increased odds of PTB, with the highest odds at the earliest gestational age PTB cutoffs. In addition, each of the 15 indicators within the 4 themes differed significantly by PTB status, suggesting that these differences were not driven by only 1 or 2 indicators within each theme.
Results in the context of what is known
Previous investigations into a broad spectrum of individual social determinants of health, including factors, such as psychosocial stress, pollution exposure, inadequate medical care, and residence in unsafe neighborhoods, have noted profound impacts on perinatal outcomes, with most studies reporting increased odds of adverse outcomes in association with these factors.7,18–25 Studies are challenging because there is no consensus “gold standard” index in evaluating neighborhood-level deprivation. The Townsend deprivation index, a commonly used social deprivation index, incorporates 4 variables—unemployment in individuals above the age of 16 years, non-car ownership, non-home ownership, and household over-crowdinghome—and thus is essentially a measure of poverty.26 In contrast, the SVI incorporates 15 variables into 4 major themes. A systematic review and meta-analysis of neighborhood deprivation and adverse perinatal outcomes, including articles published throughout 2012 included 7 studies and 2,579,032 pregnancies—many of which used the Townsend deprivation index—reported an increased odds of PTB for individuals in the most deprived neighborhood quintile (OR, 1.23; 95% CI, 1.18–1.28).27 It is likely that the more comprehensive nature of the SVI compared with the Townsend deprivation index and other scales used in studies included in the meta-analysis at least partly explain the differences in our findings compared with this previous study, specifically, the magnitude of the ORs. Furthermore, the SVI provided a more thorough assessment of the community where women live, work, and recreate, and our study design permitted us to incorporate detailed, patient-level data. In contrast to multiple previous studies that relied solely on birth certificate data or ICD-9 or ICD-10 codes for diagnosis, our comprehensive database integrated electronic medical record “problem list” searches, free text data mining, and manual data verification, all steps that increased the integrity of the data.
Clinical implications
Although clinicians know that low socioeconomic status is associated with an elevated risk of PTB and other adverse perinatal outcomes, it is challenging to know which specific metrics of socioeconomic status are most pertinent. In addition, it is challenging to consider one’s neighborhood environment when assessing perinatal risk. The SVI is publicly available and provides a comprehensive “summed” assessment of the neighborhood environment and may be used when caring for individual patients to better inform the clinician regarding each individual’s neighborhood environment.
In addition to risk stratification and identification of individuals at the highest risk, this study suggested that the SVI can be used to determine which communities may benefit from specific areas of social support, which may have a “dominolike” effect in the reduction of PTB. For example, living in a community with a lower percentage of high school graduates was associated with PTB. Although this is only 1 component of the SVI, it is possible that an improvement in this metric might have a “snowball-like” effect. For example, we hypothesized that if local efforts were made to assist residents in earning their high school equivalency, a greater number of jobs and better quality jobs might be available to residents, decreasing unemployment and reducing the percentage of individuals below the poverty line, living in mobile homes, or without a vehicle in their household, which are other elements of the SVI. However, further research is required to determine which themes and subthemes are most impactful for reducing PTB and how improvement in 1 area might impact other community factors.
Research implications
This study has multiple implications moving forward as researchers strive to determine the best metric to evaluate socioeconomic status and social vulnerability factors. Future research directly comparing different metrics and indices to help determine the optimal assessment of an individual’s neighborhood during pregnancy will provide a basis to investigate whether specific community interventions—for example, increasing the education of adults (as described above) or supporting single parents may improve birth outcomes.
Similar to previous studies evaluating prematurity risk factors, we found that women identifying as Black were more likely to deliver before term.28,29 In addition, regression models, including Black race as a covariate had notably higher associations between SVI scores and PTB at each gestational age cutoff. The relationship between race and adverse birth outcomes is complex, and a detailed discussion of this multifactorial issue is beyond the scope of this paper. Women of color who are impacted by overt and structural racism, often resulting in inequitable systems of housing, education, employment, healthcare, criminal justice, and so on, have higher risks of PTB. We assumed that the differences noted in our regression models when Black women were excluded from these models reflected unmeasured confounding that is challenging to capture, including the aforementioned factors and possibly other factors, such as environmental exposures, epigenetic modifications, or other genetic polymorphisms.30,31 Ultimately, we have acknowledged that Black race in the context of this study serves as a surrogate for racism and health inequities and is not a reflection of Black race as a “disease.” However, it remains difficult to determine the reasons for these findings; this is an important area warranting additional research.
Strengths and limitations
This study has multiple strengths. Our large sample size spanned 784 census tracts, representing a large, geographically diverse area, increasing the generalizability of these findings. Our detailed clinical data enabled the incorporation of both patient-level and community-level factors. These findings will be added to the growing body of literature supporting the expanded utility of the SVI beyond the assessment of emergency preparedness. The application of this SVI to objectively assess which specific components of social vulnerability carry the highest association with PTB has direct applicability beyond this large North Carolina cohort. Because the SVI is easily accessible and publicly available, it can be readily applied to other areas across the United States.
These data should be interpreted within the context of the study limitations. It is important to note that this study did not identify any specific census tract or area that has “high” social vulnerabilities. In addition, we chose not to dichotomize or create categorical variables for the SVI scores (except for the Kaplan-Meier survival analysis where 2 groups were necessary) because these groupings would be very population specific and limit generalizability. Our findings suggested an association between a higher SVI and prematurity but do not establish causality. It is also unlikely that this tool captures all of the community factors relevant to health or perinatal outcomes. In addition, we did not separately evaluate spontaneous PTB and medically indicated PTB as these complications are competing outcomes. Furthermore, it is unclear whether these results would be generalizable to a lower risk study population. Finally, we are unable to determine whether individuals in this cohort moved addresses during the study or whether they spent most of their time at another location (eg, work or a family member’s house) with different social vulnerabilities.
Conclusions
The readiness and resiliency of a community to natural or human-made threats to public health are significant indicators of the makeup and resources of that community. Our study findings suggested that public health officials can use this existing, publicly accessible index to assess communities at the highest risk of prematurity to target these communities for policy interventions to reduce PTB. Although there are few effective therapies to reduce the risk of PTB, knowledge regarding the community in which patients reside may help providers to risk stratify women early in pregnancy and provide support and referrals to resources for social and financial assistance as necessary. Future studies should assess the effectiveness of such interventions on prematurity when implemented in areas of high social vulnerability.
AJOG MFM at a Glance.
Why was this study conducted?
Several earlier studies have evaluated the associations between individual community-level risk factors and prematurity, but it remains challenging to identify which social determinants carry the greatest risk of preterm birth (PTB). This study aimed to determine whether the Centers for Disease Control and Prevention’s Social Vulnerability Index (SVI) (a tool integrating multiple community-level risk factors into 4 main composite themes) is associated with PTB in a cohort of women at high risk of delivering prematurely.
Key findings
Women residing in an area with higher levels of social vulnerability have higher adjusted odds ratio (aOR) of PTB before 37, 34, and 28 weeks’ gestation, and their neonates have a higher aOR of composite major neonatal morbidity.
What does this add to what is known?
The SVI is a valuable tool that may further risk stratify individuals at the highest risk of PTB. Consideration of the SVI may enable clinicians to integrate information regarding an individual’s neighborhood to further refine PTB risk assessment.
Acknowledgments
This study was funded, in part, by grant numbers K24-ES031131 and R01-MD011609 from the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
The authors report no conflict of interest.
This study was accepted for oral presentation (final abstract identification number, 14) at the 41st annual meeting of the Society for Maternal-Fetal Medicine, held virtually, January 25–30, 2021.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep 2019;68:1–47. [PubMed] [Google Scholar]
- 2.Glover AV, Manuck TA. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: a review. Semin Fetal Neonatal Med 2018;23:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Sayed AM, Galea S. Temporal changes in socioeconomic influences on health: maternal education and preterm birth. Am J Public Health 2012;102:1715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol 2011;38:351–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetherington E, Doktorchik C, Premji SS, McDonald SW, Tough SC, Sauve RS. Preterm birth and social support during pregnancy: a systematic review and meta-analysis. Paediatr Perinat Epidemiol 2015;29:523–35. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman MC, Mazzoni SE, Wagner BD, Laudenslager ML, Ross RG. Measures of maternal stress and mood in relation to preterm birth. Obstet Gynecol 2016;127:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo LQ, Chen Y, Mi BB, et al. Ambient air pollution and adverse birth outcomes: a systematic review and meta-analysis. J Zhejiang Univ Sci B 2019;20:238–52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open 2020;3:e208243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Profit J, Gould JB, Bennett M, et al. Racial/ethnic disparity in NICU quality of care delivery. Pediatrics 2017;140:e20170918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horbar JD, Edwards EM, Greenberg LT, et al. Racial segregation and inequality in the neonatal intensive care unit for very low-birth-weight and very preterm infants. JAMA Pediatr 2019;173:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Agency for Toxic Substances and Disease Registry/Geospatial Research Analysis and Services Program. CDC social vulnerability index. 2018. Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html. Accessed August 1, 2020.
- 12.Centers for Disease Control and Prevention Agency for Toxic Substances and Disease Registry/Geospatial Research Analysis and Services Program. CDC social vulnerability index. 2014. Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html. Accessed August 1, 2020.
- 13.Centers for Disease Control and Prevention Agency for Toxic Substances and Disease Registry/Geospatial Research Analysis and Services Program. CDC social vulnerability index. 2016. Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html. Accessed August 1, 2020.
- 14.Zottarelli LK, Sharif HO, Xu X, Sunil TS. Effects of social vulnerability and heat index on emergency medical service incidents in San Antonio, Texas, in 2018. J Epidemiol Community Health 2021;75:271–6. [DOI] [PubMed] [Google Scholar]
- 15.Yee CW, Cunningham SD, Ickovics JR. Application of the social vulnerability index for identifying teen pregnancy intervention need in the United States. Matern Child Health J 2019;23:1516–24. [DOI] [PubMed] [Google Scholar]
- 16.Chau PH, Gusmano MK, Cheng JO, Cheung SH, Woo J. Social vulnerability index for the older people-Hong Kong and New York City as examples. J Urban Health 2014;91:1048–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Committee Opinion no. 700 summary: methods for estimating the due date. Obstet Gynecol 2017;129:967–8. [DOI] [PubMed] [Google Scholar]
- 18.Griggs KM, Hrelic DA, Williams N, McEwen-Campbell M, Cypher R. Preterm labor and birth: a clinical review. MCN Am J Matern Child Nurs 2020;45:328–37. [DOI] [PubMed] [Google Scholar]
- 19.Lumley J Defining the problem: the epidemiology of preterm birth. BJOG 2003;110 (Suppl20):3–7. [PubMed] [Google Scholar]
- 20.Moutquin JM. Classification and heterogeneity of preterm birth. BJOG 2003;110 (Suppl20):30–3. [DOI] [PubMed] [Google Scholar]
- 21.Shah PS, Shah J. Knowledge Synthesis Group on Determinants of Preterm/LBW Births, Knowledge Synthesis Group on Determinants of Preterm LBWB. Maternal exposure to domestic violence and pregnancy and birth outcomes: a systematic review and meta-analyses. J Womens Health (Larchmt) 2010;19:2017–31. [DOI] [PubMed] [Google Scholar]
- 22.Hill A, Pallitto C, McCleary-Sills J, Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. Int J Gynaecol Obstet 2016;133:269–76. [DOI] [PubMed] [Google Scholar]
- 23.Holzman C, Eyster J, Kleyn M, et al. Maternal weathering and risk of preterm delivery. Am J Public Health 2009;99:1864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love C, David RJ, Rankin KM, Collins JW Jr.. Exploring weathering: effects of lifelong economic environment and maternal age on low birth weight, small for gestational age, and preterm birth in African-American and white women. Am J Epidemiol 2010;172:127–34. [DOI] [PubMed] [Google Scholar]
- 25.Shah PS, Balkhair T. Knowledge Synthesis Group on Determinants of Preterm/LBW births, Knowledge Synthesis Group on Determinants of Preterm. Air pollution and birth outcomes: a systematic review. Environ Int 2011;37:498–516. [DOI] [PubMed] [Google Scholar]
- 26.Jarman B, Townsend P, Carstairs V. Deprivation indices. BMJ 1991;303:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vos AA, Posthumus AG, Bonsel GJ, Steegers EA, Denktas S. Deprived neighborhoods and adverse perinatal outcome: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2014;93:727–40. [DOI] [PubMed] [Google Scholar]
- 28.Krieger N, Van Wye G, Huynh M, et al. Structural racism, historical redlining, and risk of preterm birth in New York City, 2013–2017. Am J Public Health 2020;110:1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorch SA, Enlow E. The role of social determinants in explaining racial/ethnic disparities in perinatal outcomes. Pediatr Res 2016;79:141–7. [DOI] [PubMed] [Google Scholar]
- 30.Hong X, Bartell TR, Wang X. Gaining a deeper understanding of social determinants of preterm birth by integrating multi-omics data. Pediatr Res 2021;89:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burris HH, Collins JW Jr.. Race and preterm birth–the case for epigenetic inquiry. Ethn Dis 2010;20:296–9. [PubMed] [Google Scholar]


