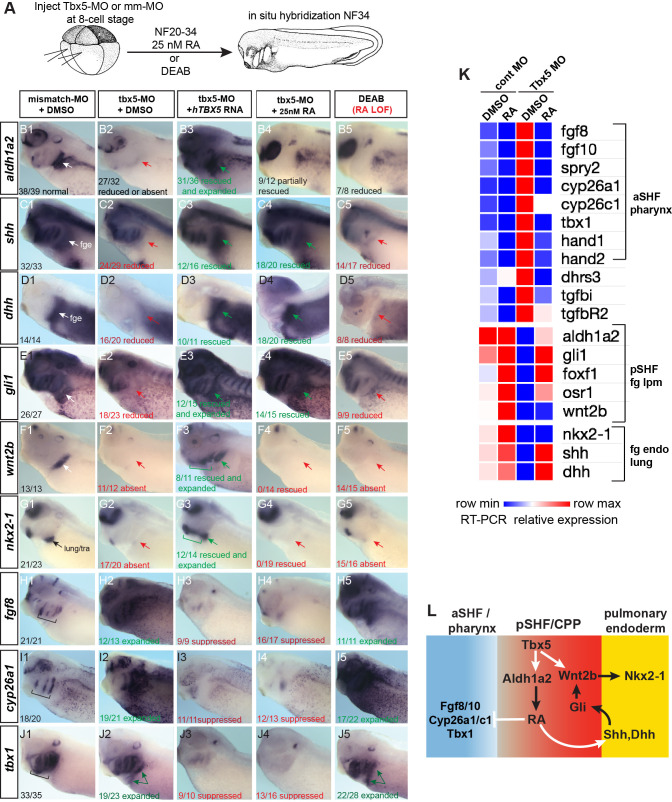
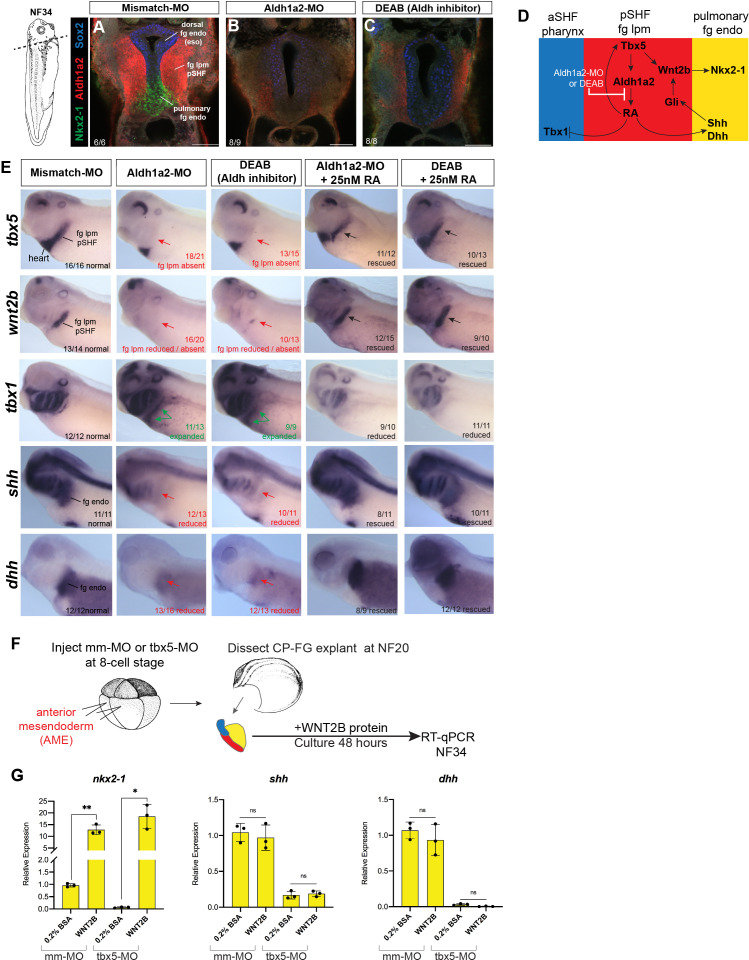
Figure 3. Tbx5 regulates Xenopus cardiopulmonary development in part via RA.
(A) Schematic of the experimental design. (B–J) Exogenous RA rescues Tbx5 LOF, while inhibition of RA phenocopies Tbx5 LOF. Whole-mount in-situ hybridization of NF34 X. laevis embryos after the indicated experimental treatments: injection of negative control 3 bp mismatch-MO (10 ng), Tbx5-MO (10 ng), Human TBX5 RNA (hTBX5; 100 pg), and/or 25 nM RA, 10 µM DEAB, DMSO vehicle control from NF20-34. The numbers of embryos with the observed expression pattern are indicated. Arrows indicate the relevant expression domain in the cardiopulmonar (CP) tissue. Brackets indicate the aSHF/pharyngeal domain. (K) Heat map showing relative expression from RT-PCR analysis of NF34 CP-foregut (fg) tissue dissected from control or Tbx5-MO injected embryos and treated with or without RA from NF20 to NF34. Each row is the average from the three biological replicates (n=4 explants per replicate). (L) Diagram of the proposed GRN model at NF25–35 showing the key role of Aldh1a2-dependent RA signaling downstream of Tbx5. White arrows indicate relationships tested in the above experiments and black arrows are demonstrated from the previous publications. Also see Figure 3—figure supplement 1, and related source data files. GRN, gene regulatory network; LOF, loss-of-function; MO, morpholino; RA, retinoic acid.