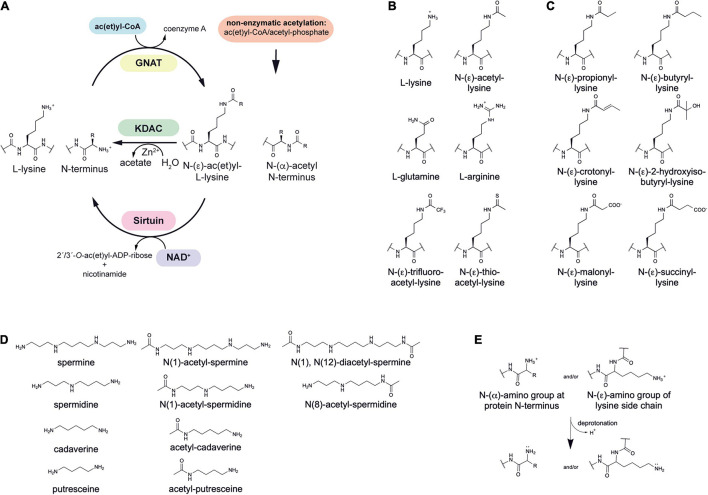
FIGURE 1.
Post-translational lysine ac(et)ylation is dynamically regulated by lysine deac(et)ylases and N-(α)-/N-(ε)-ac(et)yltransferases. (A) The ε-amino group of lysine side chains and the α-amino group at the protein N-termini can be ac(et)ylated by lysine acetyltransferases (KATs) using acetyl-CoA and/or further acyl-CoA donor molecules for the ac(et)ylation. So far, all bacterial protein acetyltransferases belong to the Gcn5-related N-terminal acetyltransferases (GNAT). Next to the enzymatic ac(et)ylation, lysine side chains and protein N-termini can ac(et)ylated non-enzymatically by ac(et)yl-CoA and acetyl-phosphate, the major source for non-enzymatic acetylation in bacteria. Bacteria use Zn2+-dependent classical lysine deac(et)ylases and NAD+-dependent sirtuin deac(et)ylases to catalyze the deac(et)ylation of lysine side chains. (B) Structures of amino acids used to study lysine acetylation. L-glutamine is often used to mimic lysine acetylation and L-arginine to conserve a non-acetylated state in studies performed in vivo. Trifluoroacetyl-L-lysine and thioacetyl-L-lysine are used as mechanistic inhibitors for sirtuins to stabilize the acetylation at an analyzed site. Notably, these analogs can be potently deacetylated by some classical deacetylases. (C) Diverse acylations identified to occur at lysine side chains in bacteria. Lysine side chains can be modified by various aliphatic or negatively charged acylations in bacteria. Further acylations might be discovered in future. To which extend acetyltransferases are capable to catalyze acylation of the lysine side chains and/or protein N-termini needs further investigation. In general, all acyl-CoA thioesters generated in metabolism can be transferred to the ε-amino group of lysine side chains and/or the α-amino group at the protein N-termini in terms of an non-enzymatic reaction. (D) Polyamines in bacteria shown to be acetylated by acetyltransferases and deacetylated by classical deacetylases. These polyamines might form buffers for acetyl-groups to avoid systemic non-enzymatic protein acetylation. The acetyl-groups can be transferred from acetyl-CoA by the action of polyamine specific acetyltransferases. (E) Increasing the reactivity at the N-(ε)-amino group of lysine side chains or the N-(α)-amino group of the protein N-termini for enzymatic or non-enzymatic ac(et)ylation. Deprotonation of the α- or ε-amino groups by protein acetyltransferases in an important step for acetyl-group transfer from the ac(et)yl-CoA donor molecule during catalysis. A deprotonation can also occur non-enzymatically and is supported by the presence of the lysine side chain in a poly-basic sequence context resulting in the reduction of the substrate lysine side chain’s pKa value. Moreover, non-enzymatic ac(et)ylation is preferred under alkaline conditions and under high concentrations of the reactive ac(et)yl-CoA thioesters. A deprotonation of the substrate amino group results in an increase in its nucleophilicity for attack of the ac(et)yl-CoA thioesters.