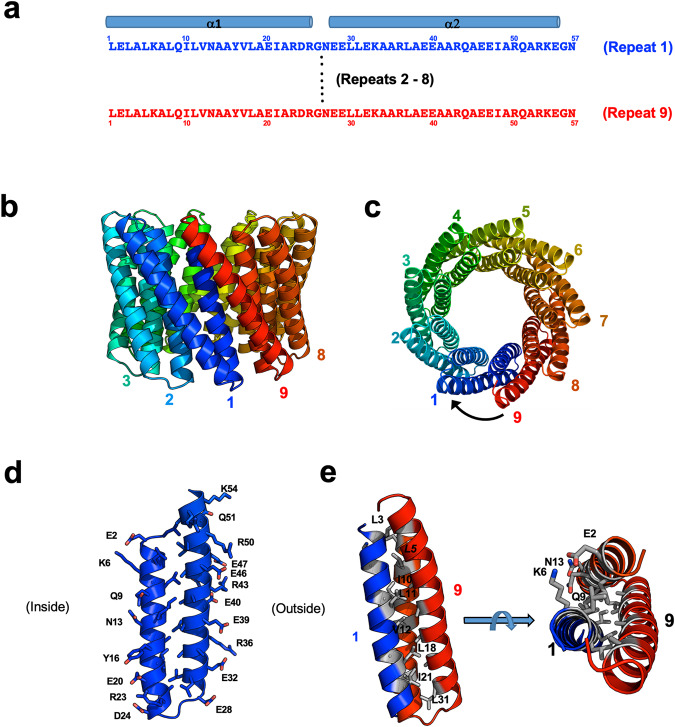
Fig. 1. Design of tcTRP9.
a Sequence and secondary structure of individual repeats, which each consist of an anti-parallel two-helix bundle connected by short turns composed of ‘GN’ sequences. b, c Nine consecutive repeats (colored blue at the N-terminal repeat and progressing to red at the C-terminal repeat in all figure panels) form a closed cylindrical structure, with an exterior diameter of ~60 Å and an interior pore diameter of ~15 Å. d The structural composition of the two-helix bundle corresponding to each designed repeat places mostly charged side chains (largely glutamic acid and arginine residues) on the exterior of the particle, and a network of charged and hydrophilic residues (along with one aromatic tyrosine) on its interior surface. The interface between the two helices is largely composed of a series of leucine and alanine residues (not labeled). e The interface between individual repeats corresponds to a series of beta-branched (Ile and Val) and gamma-branched (Leu) hydrophobic residues that form a hydrophobic core between helices, flanked by hydrophilic residues extending towards the interior and exterior of the tcTRP particles.