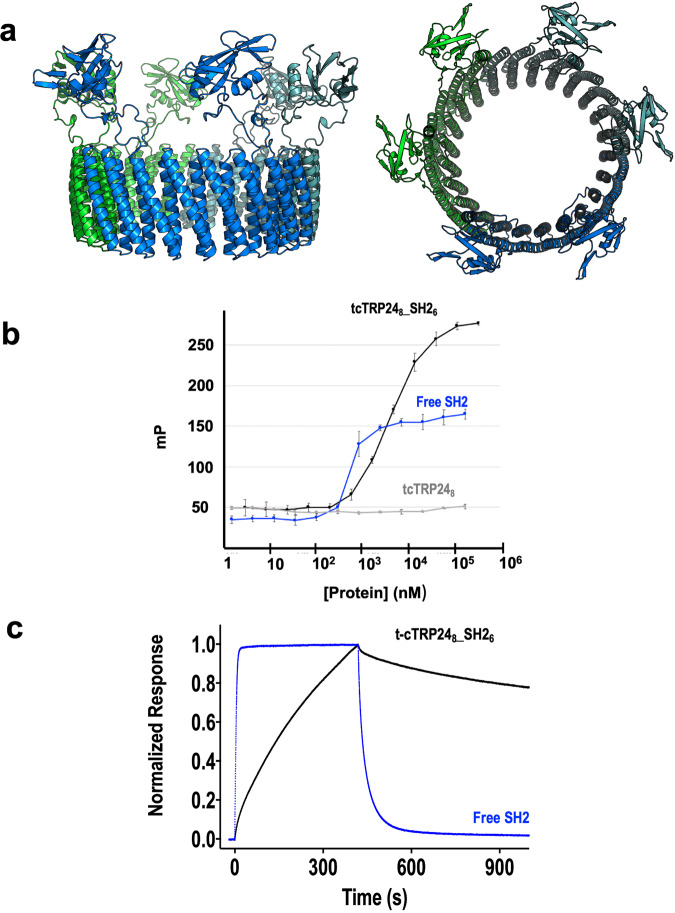
Fig. 7. Peptide binding function of tcTRP248SS_SH26 in solution and on a surface.
a Schematic model of the construct shown in two orientations. Two copies of the SH2 domain are fused into a loop (between repeats 2 and 3, and between repeats 6 and 7) in each of three subunits, resulting in the presence of six domains distributed around the periphery of the construct in a hexagonal arrangement. b Binding of fluorescently labeled Tir10 phosphotyrosyl peptide (corresponding to the physiological binding target for the Nck SH2 domain) was measured in solution using fluorescence polarization. Binding activity was measured using isolated SH2 domain (blue), and tcTRP248-SH22 (a tcTRP trimer containing two copies of fused SH2 domains per subunit, i.e., six copies total; black). A control experiment was also conducted using the free ‘naked’ tcTRP248 (gray) that contained no functional protein domains. The protein concentration is normalized to account for the ratio of SH2 domains per molecule (6 SH2 domains per tcTRP8_SH22 versus 1 per free SH2). Both constructs display saturable binding and an approximate KD of 1−3 micromolar. All binding experiments were conducted in triplicate using independent aliquots of each protein. Data shown as mean and standard deviation for n = 3 measurements. c Binding and dissociation of free SH2 (blue) and cTRP248-SH22 (black) to surface-bound peptide. Surface Plasmon Resonance (‘Biacore’) was used to measure the binding to captured biotinylated phosphotyrosyl peptide as described in Methods.