Figure 3.
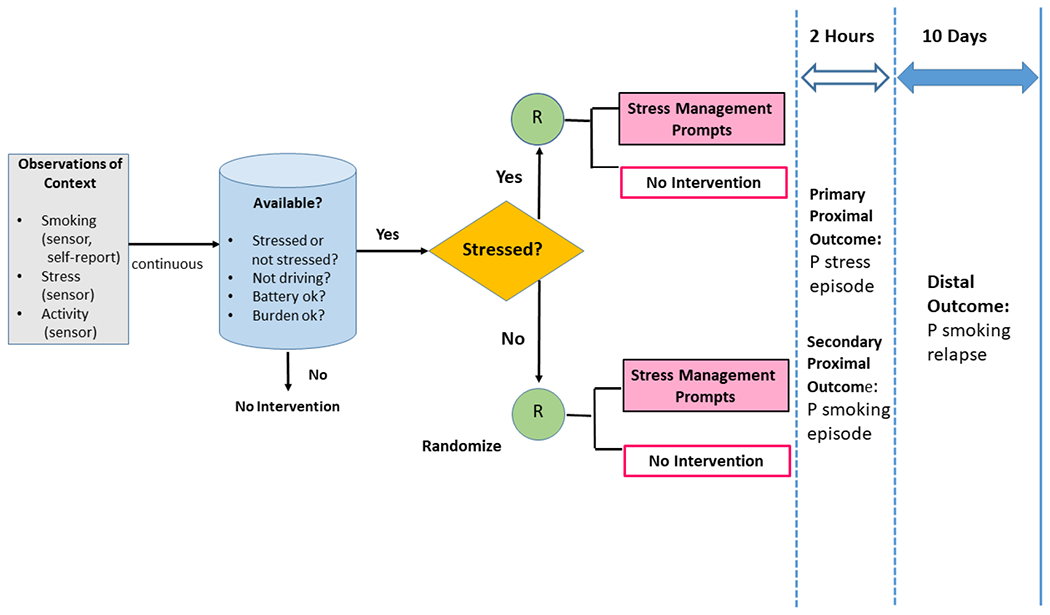
Design of the Sense2Stop Micro-Randomized Trial.
Depicts assessment modalities (sensor, self-report) used to observe Sense2Stop proximal outcomes (smoking, stress) and confounders (physical activity). Algorithms operating on the mobile phone transmit episodes of continuous digital data for micro-randomization (R) to intervention or no intervention only when the participant is available and the episode is able to be classified as probably stressed or probably not stressed. Proximal outcomes (probabilities of stress and smoking) are assessed in the 2-hour window after each micro-randomization. Note that the 10-day distal outcome is not being directly assessed in either the primary or secondary analytic plans outlined in the current manuscript.
