Figure 3.
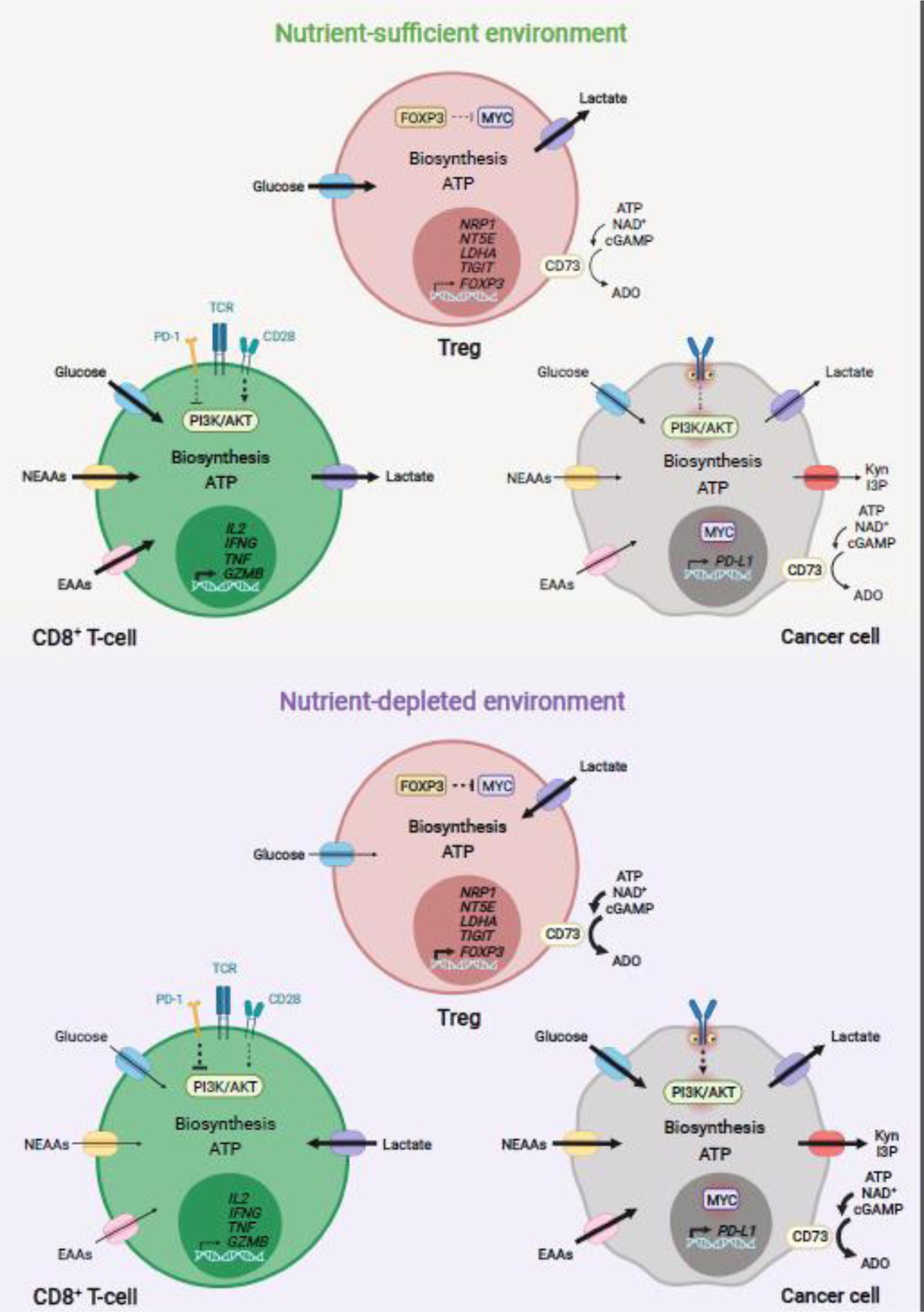
Model of metabolic modulation of antitumor immunity in the TME. In human and mouse, glucose, NEAAs and EAAs are essential for antitumor CD8+ T-cell proliferation, long-term survival, and effector function within tumors. Uptake and metabolism of these nutrients is largely modulated via ligand-receptor signaling and availability of extracellular nutrients in the TME [77,78]. Cancer cell nutrient uptake and metabolism comprise a main mechanism modulating antitumor responses in multiple human and mouse tumor entities [8,67,77,78,93]. Oncogenic signaling and immune-driven activation of metabolic enzymes are main drivers of nutrient uptake and metabolism in cancer cells [44,77,78,137]. In tumors where cancer cells have low nutrient avidity, nutrients are sufficiently taken up by antitumor CD8+ T-cells, enabling proliferation, survival, and effector function [67,93]. In this nutrient-sufficient environment, increased glucose uptake in Treg cells can impair their suppressive function [67,107]. Conversely, high nutrient avidity by cancer cells (e.g. highly glycolytic tumors), can decrease extracellular nutrient availability, leading to impairment of antitumor CD8+ T cell function [67,93,107]. In this nutrient-deprived TME, cancer cells might enhance the production and secretion of suppressors of antitumor CD8+ T-cells, including PD-L1 and lactate [93]. Expression of tryptophan catabolizing enzymes, by cancer and stromal cells, may further contribute to nutrient depletion and generation of immunosuppressive metabolites, including Kyn and I3P [44,55,137]. Ectonucleotidases, such as CD73, are expressed by multiple cancer cell types; Treg cells and other stromal cells may produce immunosuppressive ADO [60,121]. Increased uptake and oxidation of lactate can also support the proliferation and immunosuppressive function of Treg cells [106,107]. ADO, adenosine; CD73, cluster of differentiation 73; EAAs, essential amino acids; NEAAs, non-essential amino acids; I3P, indole-3-pyruvate; Kyn, kynurenine; PD-L1, programmed cell death 1 ligand 1; TME, tumor microenvironment; Treg, regulatory T cell. Figure created with BioRender.com
