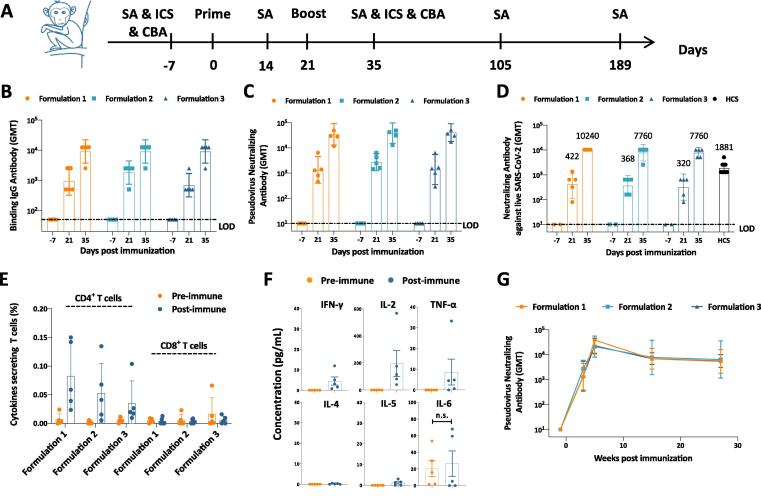
Fig. 4.
Immune responses in vaccinated cynomolgus monkeys. (A) Experiment schedule. Each group of Cynomolgus monkey (N-5) were immunized twice intramuscularly with different formulations of vaccines at Day 0 and Day 21. Formulation 1 contained 50 μg SΔTM, 500 μg Alum and 500 μg CpG; Formulation 2 contained 25 μg SΔTM, 500 μg Alum and 500 μg CpG; and Formulation 3 contained 50 μg SΔTM, 500 μg Alum and 250 μg CpG. 7 days before first immunization (Day −7), blood was collected from all individual monkeys to detect the baseline of both humoral and cellular immune responses. Blood was also collected at Day 14, Day 35, Day 105 and Day 189 to perform serological assays (SAs). In addition, PBMCs were isolated at Day 35 to detect cellular immune responses by intracellular cytokine staining (ICS) and cytometric bead array (CBA). (B) Binding antibody titers, (C) pseudovirus neutralizing antibody titers, and (D) live virus neutralizing antibody titers were evaluated at different timepoints as indicated. (E) Antigen specific T cell responses in vaccinated monkeys were determined by ICS at Day −7 (Pre-immune, as negative control) and Day 35 (Post-immune). (F) Th1-associated cytokines (IFN-γ, IL-2, TNF-α) and Th2-associated cytokines (IL-4, IL-5, IL-6) secreted from formulation 1 vaccine vaccinated monkeys were detected by CBA. (G) Pseudovirus neutralizing antibody responses were detected at Day −7, Day 14, Day 35, Day 105 and Day 189 to monitor the immune persistence. Dotted lines represent the limit of detection (LOD). Each dot represents an individual monkey. Numbers on the top of each bar represent geometric mean titers. Statistical analysis was performed using t-test with Welch’s correction. n.s.: no significance.