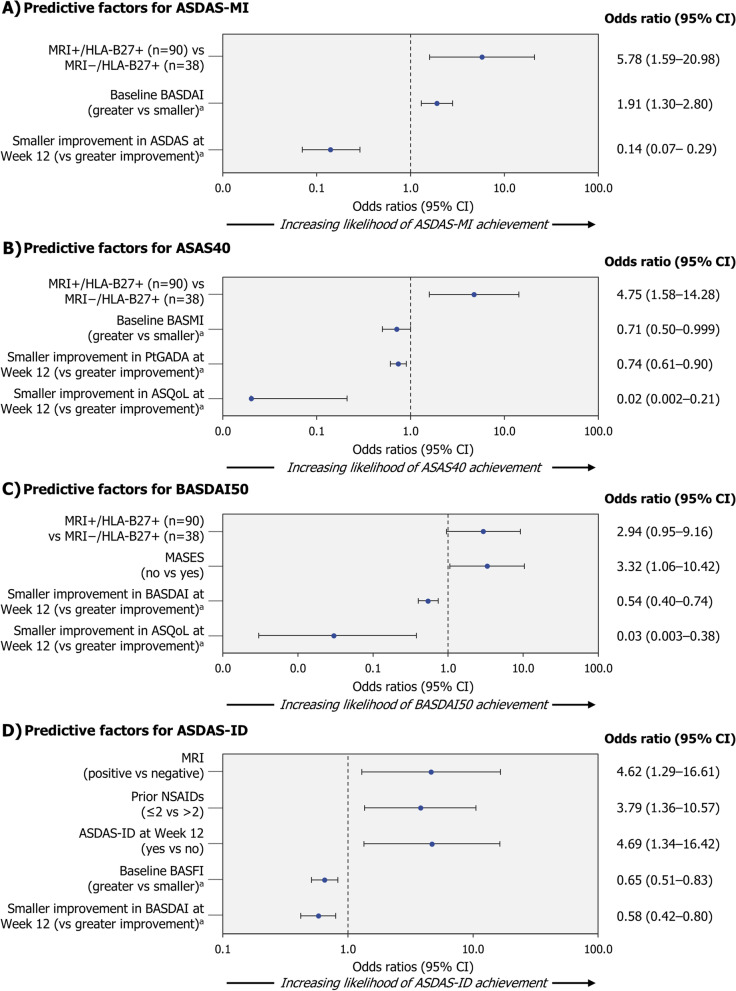
Fig. 2.
Predictive factors of Week 52 response in CZP-treated patients.
Randomised set (NRI). Patients received CZP 200 mg Q2W (400 mg loading dose at Weeks 0, 2 and 4) plus non-biologic background medication. aIncluded in the predictive model as continuous variables; for these factors, an odds ratio >1 indicates a higher probability of larger values being predictive of a response. For Week 12 change from baseline measures, a lower (negative) value is indicative of improvement, while larger (positive) values indicate worsening. ASAS40 Assessment of SpondyloArthritis International Society 40%, ASDAS Ankylosing Spondylitis Disease Activity Score, ASDAS-ID ASDAS inactive disease (ASDAS<1.3), ASDAS-MI ASDAS major improvement (reduction in ASDAS≥2.0), ASQoL ankylosing spondylitis quality of life, BASDAI50 Bath Ankylosing Spondylitis Disease Activity Index 50%, BASFI Bath Ankylosing Spondyloarthritis Functional Index, BASMI Bath Ankylosing Spondylitis Metrology Index, CI confidence interval, CZP certolizumab pegol, HLA-B27 human leukocyte antigen-B27, MRI+/− presence/absence of sacroiliitis on magnetic resonance imaging, MASES Maastricht Ankylosing Spondylitis Enthesitis Score (range 0−13), NRI non-responder imputation, NSAID non-steroidal anti-inflammatory drug, PtGADA Patient Global Assessment of Disease Activity, Q2W every 2 Weeks, vs versus