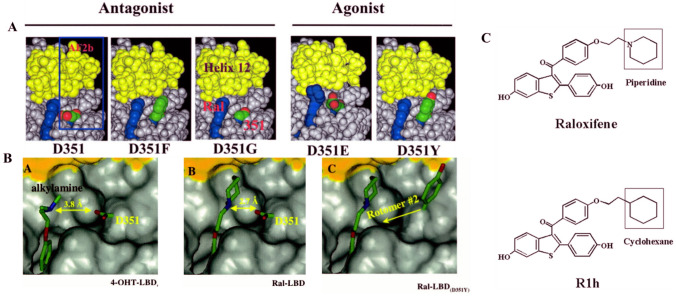
Fig. 3.
Prior to the resolution of the ligand-binding domain of the ER with either raloxifene [81] or 4-hydroxytamoxifen [80], we had identified amino acid Asp351 in the ER as the AER, i.e., the amino acid that interacts with the antiestrogenic side chain of raloxifene [103, 104]. This was followed by structure–function analyses of the ER complexes with mutations substituted at Asp351 (panels A). These were classified as retaining antiestrogenic properties or changing their actions to be estrogens (agonists). Panel 3A: Surface structures around amino acid 351 of raloxifene-bound LBDs of ERα. A structural model of dimeric human ERα bound to raloxifene was derived from the Protein Data Bank (code 1ERR) by removing all water molecules with the exception of the ordered water-forming H-bond with the O3 of raloxifene, adding hydrogens and minimizing in the consistent valence force field (CVFF) using Discover (Accelrys, San Diego, CA). Mutant receptors were constructed using Biopolymer (Accelrys) to replace Asp351 with Gly, Glu, Phe, or Tyr and to obtain a minimum energy rotomer for the mutant side chain. The results were visualized using Insight II (Accelrys). Molecular modeling of the surface structures of 4-hydroxytamoxifen-LBD (wild type) (A) or raloxifene-LBD (wild type) (B) and raloxifene-LBD (Asp351Tyr) (C). Asp351 replaced with Tyr351 in raloxifene-bound ERα LBD. To avoid steric clashes, Tyr351 is placed in a rotomer that projects the side chain upward. The side chain of Tyr351 is out of reach of the raloxifene side chain. Tyrosine residues typically lay down on the surface of proteins. In the ERR.pdb structure, small rearrangements in structure around Tyr351 are required to sterically accommodate the side chain. If this happens, the phenolic side chain would be oriented in rotomer #2. It is important to point out that the antiestrogenic N-containing side chain of tamoxifen (Fig. 3B) is further away from Asp351 than the N of raloxifene. This observation is consistent with the more estrogen-like actions of tamoxifen that results in higher blood clots and endometrial cancer than raloxifene [26, 70]; B, the piperidine side chain of raloxifene shields the charge of Asp351 and disturbs the local charge available for binding coactivators. As a result, AF1 and AF2 cannot collaborate properly, and TGF-α is silenced. C, the tyrosine at amino acid 351, changed the local charge available for coactivator binding because the piperidine can no longer shield the charge. Conformation of raloxifene-Asp351Tyr ERα to be 4-hydroxytamoxifen-ERα-like and TGFα gene is switched on. Panel C: Structures of raloxifene and the derivative R1h used in structure–function studies. Compound R1h is a raloxifene derivative that has a cyclohexane ring instead of a piperidine ring with no antiestrogenic actions. 3A from [107] and 3B from [106] with copy right permission