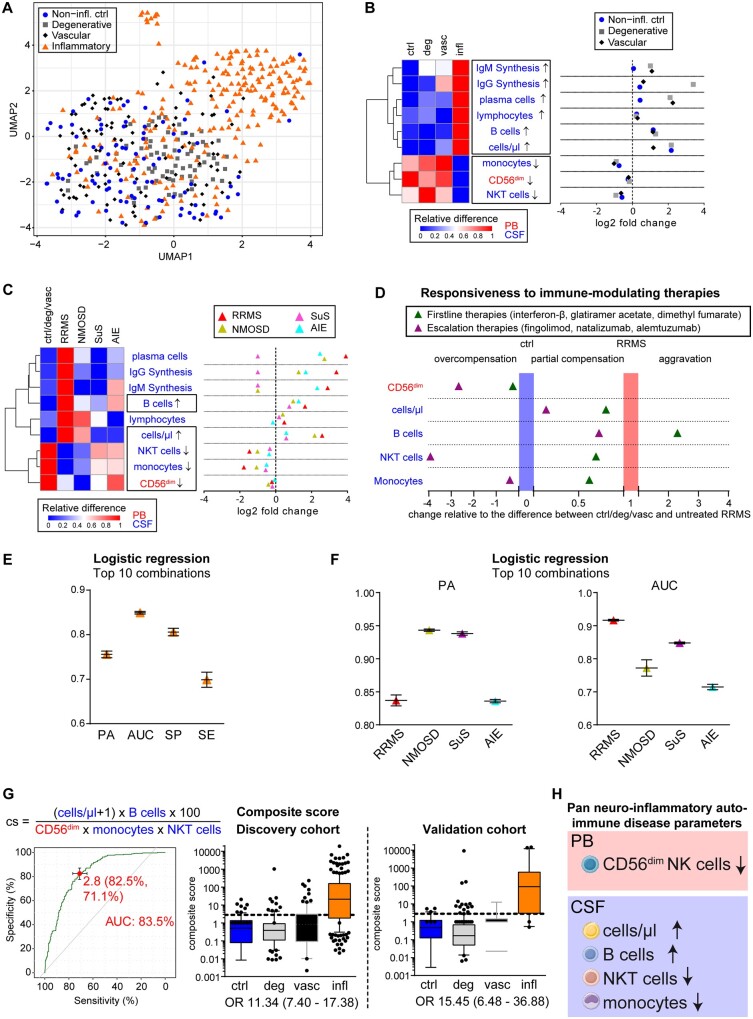
Figure 2.
Identification of pan-disease parameters characterizing CNS neuroinflammation. (A) Patients from the discovery cohort under homeostatic conditions (non-inflammatory controls, blue circle, n = 74), neurodegenerative [grey square, n = 93: amyotrophic lateral sclerosis (ALS), mild Alzheimer’s disease (mAD)], neurovascular (red diamond, n = 97) or inflammatory autoimmune CNS disorders [inflammatory, orange triangle, n = 229: Early RRMS including radiologically or clinically isolated syndrome (RIS/CIS), NMOSD, Susac syndrome (SuS) and AIE] were mapped based on immunological data from the peripheral blood and CSF following dimensionality reduction with uniform manifold approximation and prediction (UMAP). (B and C) Heat map (left) illustrating the relative changes and dot plot (right) displaying the fold changes of the nine parameters differentiating CNS neuroinflammation (infl) from non-inflammatory controls (ctrl), neurodegenerative (deg) and neurovascular (vasc) conditions (B) as well as in distinct inflammatory autoimmune diseases of the CNS (early RRMS including RIS/CIS, red triangle, n = 143; NMOSD, yellow triangle, n = 15; Susac syndrome, pink triangle, n = 14; AIE, blue triangle, n = 57) in comparison to control cohorts (C). In the heat maps, red indicates the highest expression, whereas blue indicates the lowest expression. Parameter labelling provides information on the respective compartment (peripheral blood; CSF). The black boxes highlight the commonly altered parameters. (D) Realignment plot illustrating the change in pan neuroinflammatory parameters as a consequence of first line and escalation immune therapies. The median height of each parameter was summed up and averaged as described for volcano plots by division of the result from the subtraction of the control (non-inflammatory control, degenerative, and vascular cohort) median from the respective median by the result of the subtraction of the control median from the median of treatment-naïve patients with RRMS. For CD56dim NK cells for example, controls were at 12.22%, naïve RRMS at 10.55% and escalation therapies at 17.62%, resulting in (17.62%–12.22%)/(10.55%–12.22%) = −3.23, indicating an overcompensation (<0) beyond control levels. Thus, parameters overcompensated by immunotherapy [baseline therapies (green triangle): interferon-β n = 9, glatiramer acetate n = 5 and dimethyl fumarate n = 5; escalation therapies (purple triangle): fingolimod n = 15, natalizumab n = 41 and alemtuzumab (10–14 months post last injection) n = 30 are in the left part of the graph, whereas parameters with partial compensation for neuroinflammatory alterations are in the middle. Parameters exhibiting aggravated levels more different from control cohorts than untreated RRMS patients are represented in the right of the graph. (E) Mean and standard deviation of prediction accuracy (PA), area under the curve (AUC), specificity (SP) and sensitivity (SE) from the top 10 combinations of up to five pan-disease parameters as calculated by logistic regression to differentiate all neuroinflammatory diseases from non-inflammatory controls, neurodegenerative, and neurovascular diseases. (F) Mean and standard deviation of prediction accuracy and AUC for differentiating distinct neuroinflammatory CNS diseases (RRMS, NMOSD, Susac syndrome and AIE) from control cohorts. (G) Predictive score composed by division of parameters consistently increased by parameters decreased in neuroinflammation (top). Receiver operating characteristic (ROC) analysis (left) identified a cut-off of 2.8 distinguishing (right) patients with neuroinflammatory diseases (infl) from non-inflammatory controls (ctrl), neurodegenerative (deg) and neurovascular (vasc) patients with an AUC of 83.5 and odds ratio (OR) of 11.34. This composite score was verified in the validation cohort, resulting in the identification of neuroinflammatory patients with an OR of 15.45. (H) Pan neuroinflammatory auto-immune disease parameters commonly altered in all neuroinflammatory autoimmune diseases of the CNS in comparison with other neurological diseases as well homeostatic conditions.