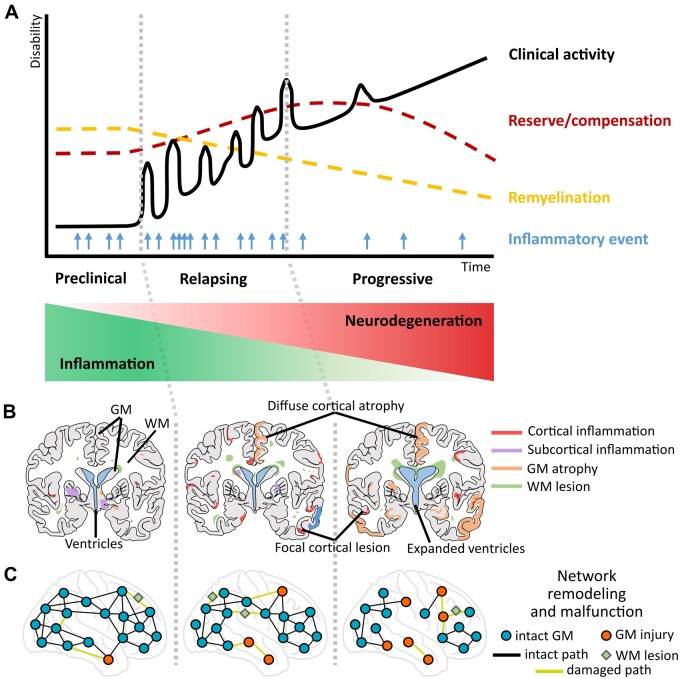
Figure 1.
An integrative hypothetical framework of the progression of multiple sclerosis pathology with evolving neurodegeneration through grey matter (GM) pathology (involving cortical and subcortical GM) that can be characterized via MRI-based network measures. (A) The y-axis of the graph shows the progression of neurological/clinical disability over time (x-axis); the black continuous line denotes clinical activity with relapsing events that cause inflammatory damage; the light blue arrows denote frequency of new inflammatory episodes from preclinical to progressive stages as recorded by MRI; the yellow dashed line denotes the slowly progressing decline in remyelination capacities of brain tissue, while more resources of reserve and compensation become necessary (red dashed line). (B) Coronary lesion maps of GM damage: cortical (red) and subcortical (purple) GM inflammation, increasing GM atrophy (orange) and white matter (WM) lesions (green) relating to distinct stages of multiple sclerosis progression. (C) Representation of network remodelling and malfunction corresponding to different stages of multiple sclerosis disease progression. Blue circles and black lines represent GM regions and their connecting paths without signs of pathology; red circles indicate GM regions affected in multiple sclerosis; green diamonds illustrate WM lesions; light green lines represent damaged connections between brain regions. Two main GM contributors are considered for this model: (i) acute or sustained focal inflammatory activity (red and purple) and (ii) continuous diffuse GM damage (atrophy; orange), both of which can indirectly but closely mirror ongoing tissue processes quantified by MRI. The network approach allows the detection of reorganization and malfunction in the cortex while highlighting its role in the transition from inflammatory demyelination to persistent neurodegeneration and progressive disease stages. This model implies that besides the primary neuroinflammatory origin of immune attacks on brain circuitry in multiple sclerosis, there is a high possibility for focal demyelination, diffuse GM tissue damage or incomplete restoration and emerging neurodegeneration to translate into altered cortical activity. The abnormal cortical activity, thus, drives the involvement of cortico-cortical and cortico-subcortical circuits, accelerates the network dysfunction and spreads secondary malfunction to the interconnected sites.