Abstract
Background
Calmodulinopathy is an emerging group of primary electrical disease with various, severe, and early onset phenotype. Sudden cardiac arrest (SCA)/death can be the first symptom and current medical management seems insufficient to prevent recurrences. Implantable cardioverter-defibrillator (ICD) in the young is challenging and can be harmful.
Case summary
We report the management of two very young boys (aged 3.5 and 5.5 years old) who survived an SCA due to calmodulin mutation responsible of a catecholaminergic polymorphic ventricular tachycardia phenotype. In both case, SCA had an adrenergic trigger. Despite SCA, ICD implantation was denied by the parents. After thorough discussion with the family, the patients were managed with solely betablocker treatment and loop recorder implantation. At last follow-up of 30 and 23 months, respectively, there were no recurrence of any cardiac event.
Discussion
The benefits of ICD implantation at a very young age must be weighed against the risk complication. In the youngest, whom recreative activities are under constant supervision, the decision, jointly made with the parents, could be to postpone ICD.
Keywords: Calmodulin, Catecholaminergic polymorphic ventricular tachycardia, Sudden cardiac death, Implantable cardioverter-defibrillator, Shared decision-making, Case report
Learning points
Catecholaminergic polymorphic ventricular tachycardia secondary to calmodulin gene mutations can cause sudden cardiac arrest (SCA) at a very young age.
Pharmacotherapy and implantable cardioverter-defibrillator (ICD) placement are indicated for secondary prevention after an SCA with an adrenergic trigger.
At the parents’ request, ICD placement can be postponed in the youngest patients, as these are under constant supervision during their waking hours.
Introduction
Calmodulinopathies are an emerging group of inherited malignant arrhythmias due to heterozygous mutations in any of the three human calmodulin (CALM) genes. The clinical presentations include long-QT syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT), idiopathic ventricular fibrillation, autopsy-negative sudden unexplained death,1 and LQTS/CPVT overlap syndrome.2 Life-threatening arrhythmias occur early in life, and current medical treatments have limited efficacy in preventing recurrences.3
Placement of an implantable cardioverter-defibrillator (ICD) is recommended in patients who experience sudden cardiac arrest (SCA) and have no evidence of reversible heart disease.2,4 However, ICDs are associated with high morbidity rates in paediatric patients.5
We describe two patients with de novo CALM mutations who experienced CPVT with successful resuscitation after SCA at a very young age and in whom a decision was made in partnership with the parents to postpone ICD therapy. The parents gave their informed consent for the reporting of these data.
Timeline
| Patient #1 | Patient #2 | |
|---|---|---|
| Birth | November 2014 | June 2013 |
| Family history of cardiac event | None | None |
| Previous symptoms in the patient | None | None |
| Date (age) at first cardiac event | May 2018 (3.5 years) | November 2018 (5.5 years) |
| Weight at first cardiac event | 18 kg | 19 kg |
| Date (age) at implantable loop recorder placement | March 2019 (4.3 years) | January 2020 (6.6 years) |
| Date (age) at last follow-up | February 2021 (6.2 years) | January 2021 (7.7 years) |
| Recurrence of cardiac event | No | No |
Case presentations
Case #1
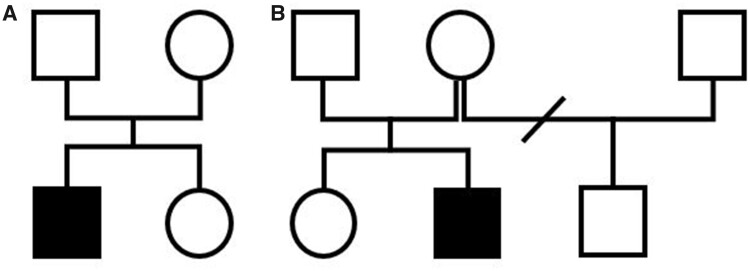
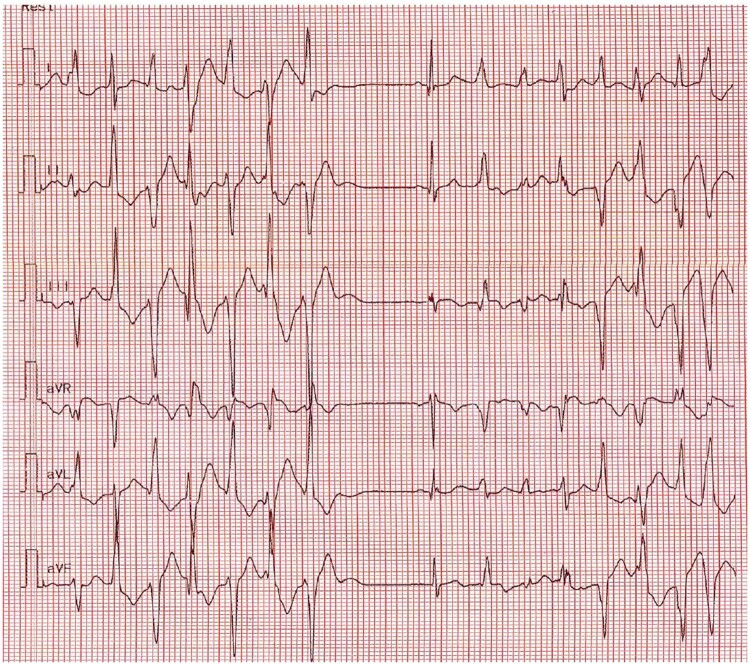
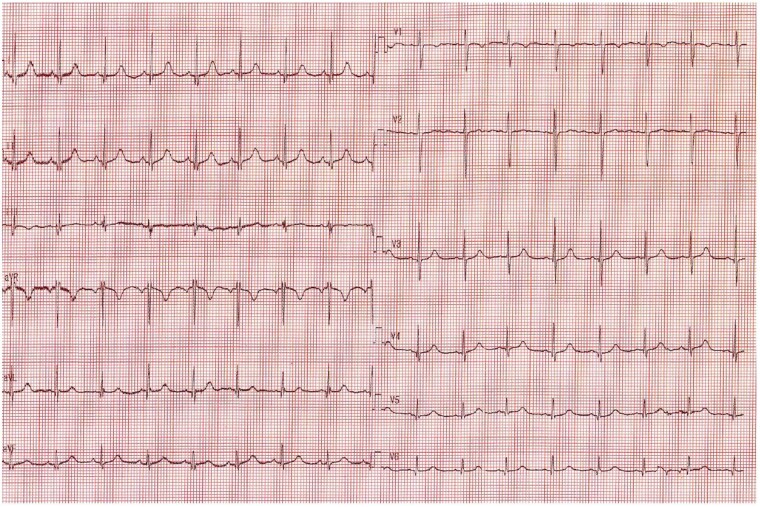
A 3.5-year-old boy with no familial or personal history of heart disease (Figure 1A) experienced severe syncope during a physical education session. Cardiopulmonary resuscitation (CPR) was immediately started by his father and restores normal pulse before emergency rescue squad intervention 15 min later. The electrocardiogram (ECG) recorded in the intensive care unit showed frequent episodes of bidirectional ventricular ectopy (Figure 2), no prominent U waves, and a normal QTc interval during sinus rhythm (Figure 3). Transthoracic echocardiography was normal. The patient weighed 18 kg at admission. Catecholaminergic polymorphic ventricular tachycardia was suspected and the patient was started on the betablocker nadolol, 40 mg/day. A heterozygous missense mutation (c.293A>G p.Asn98Ser) in CALM1 was identified in the proband but not in his parents or sister, all of whom had a normal cardiac workup. The parents refused ICD placement despite understanding the risk of recurrent SCA. After an in-depth discussion with the parents, an implantable loop recorder was placed. The parents, other relatives, and school staff received CPR training, and each was given an automated external defibrillator (AED). Nadolol therapy was continued. At last follow-up after 30 months, no further arrhythmias had occurred.
Figure 1.
Pedigrees. (A) Patient #1. (B) Patient #2.
Figure 2.
Electrocardiogram at admission showing episodes of polymorphic ventricular ectopy with frequent bidirectional ventricular doublets in Patient #1.
Figure 3.
Standard 12-lead electrocardiogram at rest after betablocker (nadolol) initiation in Patient #1: normal sinus rhythm with QTc = 420 ms.
Case #2
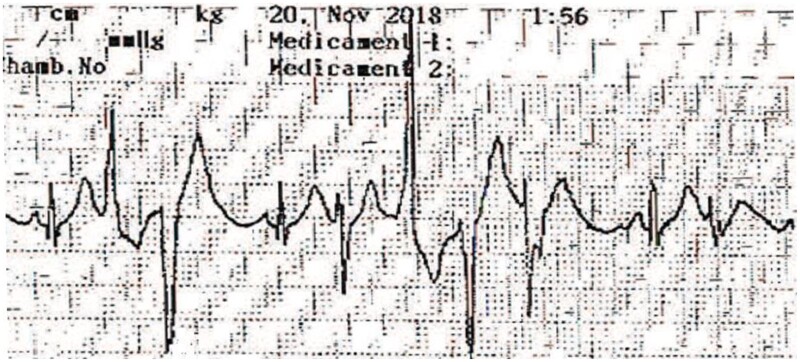
This 5.5-year-old boy experienced SCA triggered by fear and stress (Figure 1B). Cardiopulmonary resuscitation was started by the school teacher until the rescue squad intervention 20 min later. The AED detected, then, a shockable rhythm. Sinus rhythm was restored after AED shock but episodes of bidirectional ventricular ectopy were noted (Figure 4). At hospital admission, he weighed 19 kg. Transthoracic echocardiography showed no structural abnormalities or ventricular dysfunction. The ECG was normal, without ventricular ectopy and with a normal QT interval. Nadolol, 40 mg/day, was initiated immediately. Targeted gene panel sequencing revealed the same missense mutation (c.293A>G p.Asn98Ser) as in our first patient but in CALM2. The parents and older sister did not have the mutation and their cardiac workup was normal. The parents refused ICD placement due to concern about complications and despite understanding the risk of future SCA. An implantable loop recorder was placed. As with the first patient, all supervising adults received CPR training, and each was given an AED. The nadolol therapy was continued. At last follow-up after 23 months no cardiac events had occurred.
Figure 4.
Episodes of bidirectional ventricular ectopy recorded by the first responders to the sudden cardiac arrest in Patient #2.
Discussion
LQTS and CPVT are more severe when they are related to CALM mutations. Isolated CALM-related LQTS is more severe than CALM-related LQTS/CPVT overlap syndrome, which in turn is more severe than isolated CALM-related CPVT. The median age at diagnosis is 1.5 years for CALM-related LQTS and 6 years for CALM-related CPVT.3 Data in the International Calmodulinopathy Registry demonstrate that betablockers have little efficacy in LQTS/CPVT but are beneficial in LQTS type 1 and in CPVT unrelated to CALM. Severe cardiac events, including SCA, occurred despite betablocker therapy in 65% of patients with CALM-related LQTS and 36% of those with CALM-related CPVT.2 These data are reflected in current guidelines recommending ICD therapy.
Although technically challenging in young children, ICD placement is possible even in infants.6,7 The implantation technique varies with patient size and local practices. Epicardial placement is the rule in patients weighing <15–20 kg.8,9 In paediatric patients, up to 47% of ICD shocks have been inappropriate, with the main reasons being lead malfunction and oversensing.6,10 Age 5–10 years at implantation is a risk factor for epicardial ICD lead failure,9 as during this period growth is rapid and children are physically very active. Subcutaneous ICD placement may hold promise for paediatric patients, as longer lead survival compared to endocardial ICDs has been reported.11 However, ICDs are too bulky to be implanted subcutaneously in very young patients.
Whether ICD placement benefits patients with CPVT is controversial, even when the device is used for secondary prevention. Implantable cardioverter-defibrillator shocks in this population can be ineffective and potentially proarrhythmic.12–18 ICD therapy has limited ability to terminate ventricular tachycardia associated with CPVT but is more effective in stopping ventricular fibrillation.13,14 Overall, only 57% of appropriate ICD shocks terminated ventricular arrhythmias in 24 retrospectively reviewed cases.13 Moreover, inappropriate shocks can trigger electrical storms.17,18 In a study of 136 patients who were diagnosed with CPVT after experiencing SCA, the 4-year cardiac event rate was 46.8% in the 79 patients with ICDs and 15.8% in the 57 patients without ICDs, yielding a five-fold relative risk of cardiac events in the ICD group.19 Implantable cardioverter-defibrillator placement did not improve survival and was associated with a high complication rate. The authors concluded that Class I ICD placement for patients with CPVT that was undiagnosed and untreated at the time of the first cardiac event should not be considered mandatory.19
The benefits of ICD placement in patients with CPVT unrelated to CALM remain uncertain. In contrast, severe CALM-related CPVT may benefit from ICD therapy. Current data also support left cardiac sympathetic denervation (LCSD) in patients with recurrent symptoms or numerous ICD shocks despite betablocker therapy. Left cardiac sympathetic denervation is also indicated in patients with contraindications or intolerance to betablockers. Left cardiac sympathetic denervation has been used as monotherapy in selected patients with LQTS20 and might be considered an alternative to ICD in some cases, notably due to the high ICD-related morbidity in very young patients. In both our patients, given these data and after full information of the parents, we and the parents decided to postpone ICD implantation without performing LCSD. This decision was supported by the initial presentation with CPVT, the age of the patients, the constant supervision provided to young children, and the potential complications of ICD placement. Cardiopulmonary resuscitation training and an AED were provided to all supervising adults. We see these patients at three monthly intervals to assess betablocker tolerance based on the physical examination, reinforce parent training, and obtain a Holter recording. Continuous telemonitoring is achieved via the implantable loop recorder. The parents and legal guardian agree the ICD placement would be in order IF another cardiac event occur or when the boys reach their early teens.
Conclusion
Calmodulinopathies are increasingly recognized as primary electrical diseases exposing infants and young children to life-threatening cardiac arrhythmias. The calmodulin genes should be screened, especially in paediatric patients with severe LQTS, CPVT, or LQTS/CPVT overlap syndrome.
Lead author biography

Alice Maltret, MD, is a paediatric cardiologist with expertise in inherited arrhythmia. She was trained in Necker-Enfants Malades in Paris and is now working at the hospital Marie Lannelongue.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Anderson JH, Tester DJ, Will ML, Ackerman MJ.. Whole-exome molecular autopsy after exertion-related sudden unexplained death in the young. Circ Cardiovasc Genet 2016;9:259–265. [DOI] [PubMed] [Google Scholar]
- 2. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB. et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:1677–1749. [DOI] [PubMed] [Google Scholar]
- 3. Crotti L, Spazzolini C, Tester DJ, Ghidoni A, Baruteau A-E, Beckmann B-M. et al. Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur Heart J 2019;40:2964–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015;17:1601–1687. [DOI] [PubMed] [Google Scholar]
- 5. Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI.. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol 2004;15:72–76. [DOI] [PubMed] [Google Scholar]
- 6. Berul CI, Van Hare GF, Kertesz NJ, Dubin AM, Cecchin F, Collins KK. et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol 2008;51:1685–1691. [DOI] [PubMed] [Google Scholar]
- 7. Schneider AE, Burkhart HM, Ackerman MJ, Dearani JA, Wackel P, Cannon BC.. Minimally invasive epicardial implantable cardioverter-defibrillator placement for infants and children: an effective alternative to the transvenous approach. Heart Rhythm 2016;13:1905–1912. [DOI] [PubMed] [Google Scholar]
- 8. Berul CI. Defibrillator indications and implantation in young children. Heart Rhythm 2008;5:1755–1757. [DOI] [PubMed] [Google Scholar]
- 9. Radbill AE, Triedman JK, Berul CI, Fynn-Thompson F, Atallah J, Alexander ME. et al. System survival of nontransvenous implantable cardioverter-defibrillators compared to transvenous implantable cardioverter-defibrillators in pediatric and congenital heart disease patients. Heart Rhythm 2010;7:193–198. [DOI] [PubMed] [Google Scholar]
- 10. Korte T, Koditz H, Niehaus M, Paul T, Tebbenjohanns J.. High incidence of appropriate and inappropriate ICD therapies in children and adolescents with implantable cardioverter defibrillator. Pacing Clin Electrophysiol 2004;27:924–932. [DOI] [PubMed] [Google Scholar]
- 11. Pettit SJ, McLean A, Colquhoun I, Connelly D, McLeod K.. Clinical experience of subcutaneous and transvenous implantable cardioverter defibrillators in children and teenagers. Pacing Clin Electrophysiol 2013;36:1532–1538. [DOI] [PubMed] [Google Scholar]
- 12. Roses-Noguer F, Jarman JWE, Clague JR, Till J.. Outcomes of defibrillator therapy in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2014;11:58–66. [DOI] [PubMed] [Google Scholar]
- 13. Miyake CY, Webster G, Czosek RJ, Kantoch MJ, Dubin AM, Avasarala K. et al. Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ Arrhythm Electrophysiol 2013;6:579–587. [DOI] [PubMed] [Google Scholar]
- 14. Marai I, Khoury A, Suleiman M, Gepstein L, Blich M, Lorber A. et al. Importance of ventricular tachycardia storms not terminated by implantable cardioverter defibrillators shocks in patients with CASQ2 associated catecholaminergic polymorphic ventricular tachycardia. Am J Cardiol 2012;110:72–76. [DOI] [PubMed] [Google Scholar]
- 15. Roston TM, Vinocur JM, Maginot KR, Mohammed S, Salerno JC, Etheridge SP. et al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015;8:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohamed U, Gollob MH, Gow RM, Krahn AD.. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm 2006;3:1486–1489. [DOI] [PubMed] [Google Scholar]
- 17. Pizzale S, Gollob MH, Gow R, Birnie DH.. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:1319–1321. [DOI] [PubMed] [Google Scholar]
- 18. Palanca V, Quesada A, Trigo A, Jiménez J.. [Arrhythmic storm induced by AICD discharge in a patient with catecholaminergic polymorphic ventricular tachycardia]. Rev Esp Cardiol 2006;59:1079–1080. [DOI] [PubMed] [Google Scholar]
- 19. van der Werf C, Lieve KV, Bos JM, Lane CM, Denjoy I, Roses-Noguer F. et al. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J 2019;40:2953–2961. [DOI] [PubMed] [Google Scholar]
- 20. Niaz T, Bos JM, Sorensen KB, Moir C, Ackerman MJ.. Left cardiac sympathetic denervation monotherapy in patients with congenital long QT syndrome. Circ Arrhythm Electrophysiol 2020;13:e008830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.