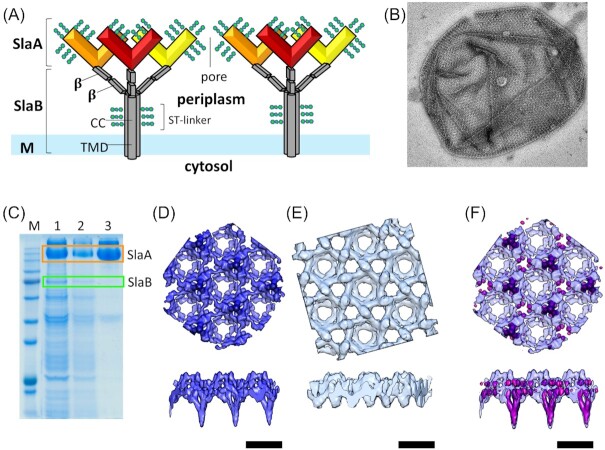
Figure 9.
Model of the Sulfolobales S-layer. (A) The Sulfolobales S-layer consists of the two protein subunits. SlaA dimers (red, orange, yellow) form the outer S-layer canopy. Each SlaA protein is predicted to be rich in β-strands. The SlaA dimer has a boomerang-like shape, the angle of which determines the S-layer unit cell size. SlaB trimers (gray) form the membrane anchors of the S-layer. Each SlaB is predicted to consist of an N-terminal transmembrane domain (TMD), a coiled-coil domain (CC) and two to three C-terminal β-sandwich domains (β). SlaA and SlaB proteins are highly glycosylated (green). (B) Electron microscopy image from negatively stained isolated S-layer from S. acidocaldarius. (C)SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) of the isolation of S-layer from S. acidocaldarius cells using detergent buffers. Lane 1: pellet after incubating the cells with the detergent buffer the first time, the second time (lane 2) and the third time (lane 3). SlaB is being successively washed off and in the last wash a pure SlaA prep is obtained. (D) Subtomogram average of fully assembled S-layer. (E) Subtomogram average of SlaB-depleted S-layer. (F) Difference map (pink) overlaid with the complete S-layer visualizes location of SlaB. (Scale bars, and C–E, 20 nm). (Figure adapted from Gambelli et al. 2019).