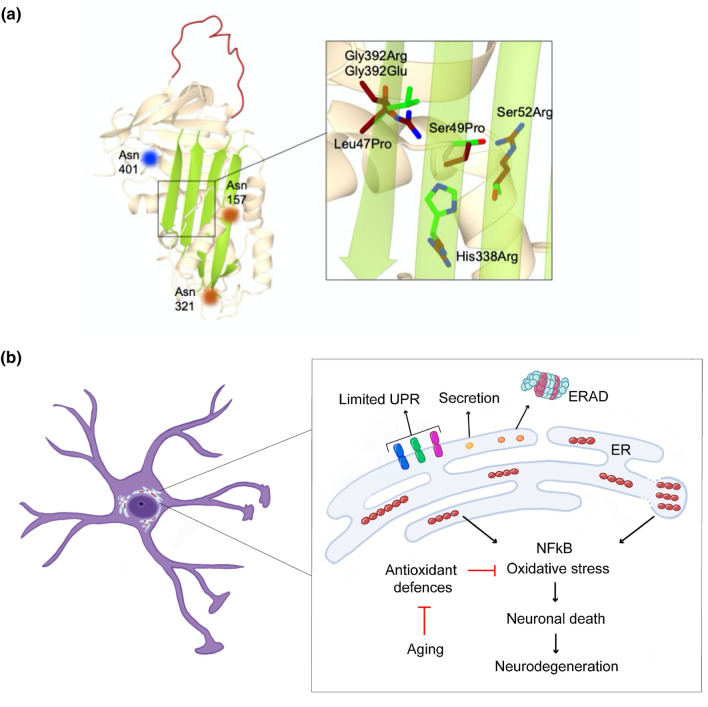
Fig. 4.
N-glycosylation of neuroserpin, FENIB related mutations and toxicity mechanisms of polymerogenic neuroserpin. a The positions of the two physiological N-glycosylation sites Asn157 and Asn321 (orange spots) and the aberrant site Asn401 (blue spot) are shown on the structure of human native neuroserpin (left panel, PDB 3F5N). The box on the right focuses on β-sheet A to show the six pathological mutations known to cause FENIB, with the wild type and mutated residues colored in green and dark red, respectively. b Cellular responses to the presence of polymerogenic mutant neuroserpin. The monomeric forms are in part secreted, in part degraded by the proteasome through ERAD and in part incorporated into polymeric chains that can be found in tubular ER and ER-derived inclusions. The expression of polymerogenic neuroserpin causes NFκB activation and chronic oxidative stress, leading to neuronal death and neurodegeneration. This is probably more pronounced with aging, due to a weakening of the antioxidant defences