FIGURE 1.
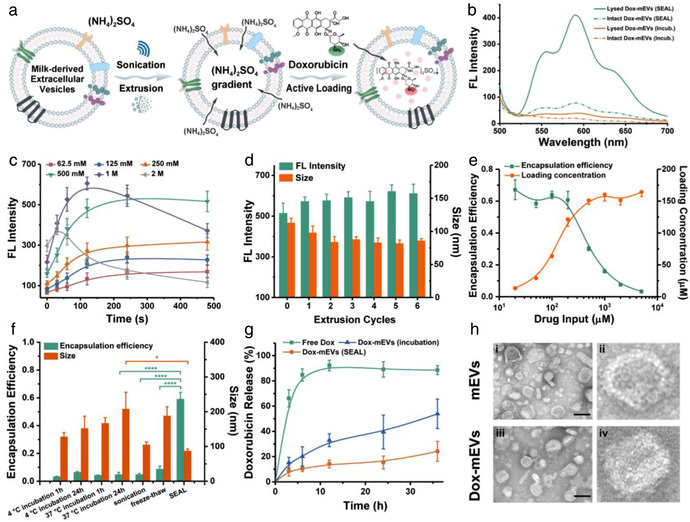
Preparation and analysis of Dox‐mEVs. (a) Schematic illustration representing the principle of SEAL. (b) Fluorescence analysis of Dox‐mEVs prepared by passive incubation and SEAL. (c) Fluorescence intensity of Dox‐mEVs at different ammonium sulfate concentration and sonication period (n = 3, mean ± SD). (d) Fluorescence intensity and particle size of Dox‐mEVs at various extrusion cycles (n = 3, mean ± SD). (e) Encapsulation efficiency and loading concentration of Dox‐mEVs at various drug input (n = 3, mean ± SD). (f) The encapsulation efficiency and particle size of Dox‐mEVs by various loading methods. p‐Values were calculated by t‐test. *p < 0.05, ****p < 0.0001 (n = 3, mean ± SD). (g) Drug release profile of free doxorubicin, as well as Dox‐mEVs prepared by SEAL and passive incubation, respectively (n = 3, mean ± SD). (h) TEM images of the unloaded mEVs (i) and SEAL‐prepared Dox‐mEVs (iii, scale bar = 100 nm). (ii) and (iv) depict a single mEV selected from the same image of (i) and (iii), respectively
