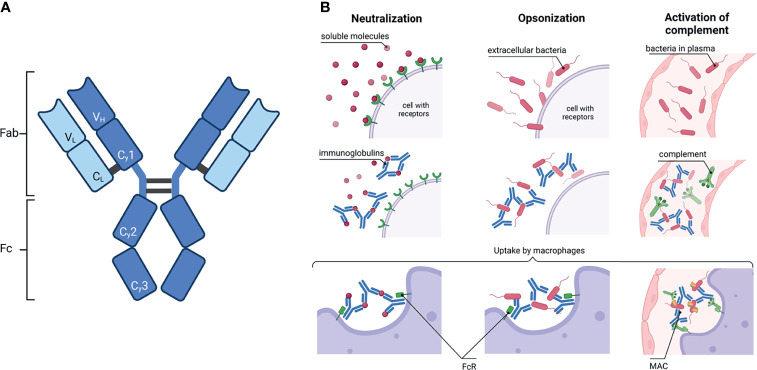
Figure 1.
Immunoglobulin: schematic structure and how they activate the immune system. (A) A common IgG structure consisting of two heavy chain (dark blue) with three constant (Cγ1-3) and one variable domain (VH) and two light chain (light blue) with one constant (CL) and one variable domain (VL). The heavy and light chains are covalently connected by disulfide bonds. The IgG is further subdivided in the antigen binding fragment (Fab) and fragment crystallisable (Fc). (B) Activation of the immune system by antibodies. Left column: Ig can neutralize soluble molecules, e.g. bacteria toxins, to protect endogenous cells. Middle column: The binding of Ig to virus or bacteria antigens is called opsonisation. Right column: Ig bound to pathogens can activate the complement. Complement factors C1q recognize Ig and induce the complement cascade; a membrane attack complex (MAC) is formed in the end. In the end, macrophages or neutrophils phagocytose the complex of Ig with either soluble molecules or bacteria or, additionally, with the components of the complement. Created with BioRender.com.