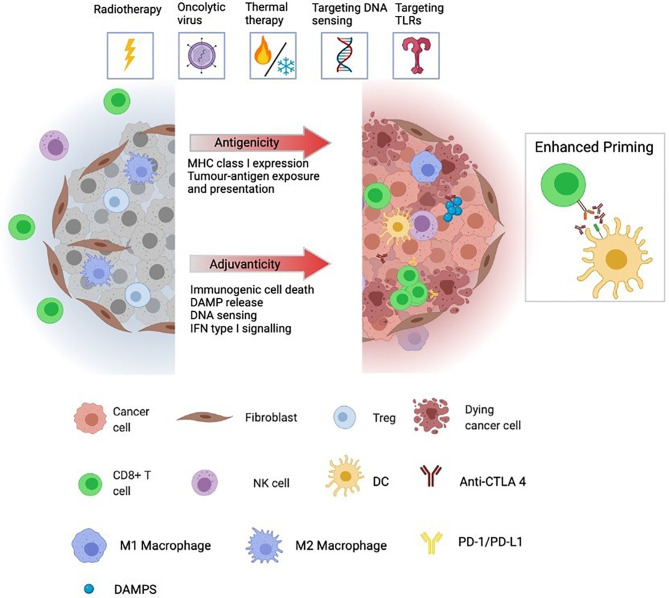
Figure 1.
Immunologically “cold” tumours are generally unresponsive to ICPI and characterised by low infiltration and/or exclusion of cytotoxic lymphocytes, including CD8 T cells and NK cells. Further, cold tumours often have high infiltration of immunosuppressive cells including Tregs, CAFs, and M2-polarized macrophages as well as low expression and presentation of tumour neoantigens preventing priming of de novo immune responses. Immunogenic localised therapies are designed to convert ‘cold’ tumours to a ‘hot’ by altering the adjuvanticity and antigenicity of the TME. Antigenicity is achieved by augmented expression, degradation and presentation of tumour neoantigens while adjuvanticity is associated with elevated levels of DAMPs, released from dying tumour cells, cytosolic DNA accumulation and sensing, and a transcriptional profile geared towards IFN type I signalling. Together, these factors promote recruitment, infiltration and activation of DCs allowing for increased antigen cross-presentation and priming of tumor-specific CD8 T cells. Triggering of these events by localised therapies creates a favourable environment for synergy with ICPI. The pool of activated cross-presenting DCs cooperates with anti-CTLA-4 treatment to generate a broadened repertoire of tumour neoantigen-specific T cells whose effector function can be augmented by anti-PD-1 treatment in their killing of tumour cells locally and systemically. TME, tumour microenvironment; DAMP, danger-associated molecular pattern; DC, dendritic cell; CAF, cancer-associated fibroblast; ICPI, immune checkpoint inhibitor; NK cell, natural killer cell.