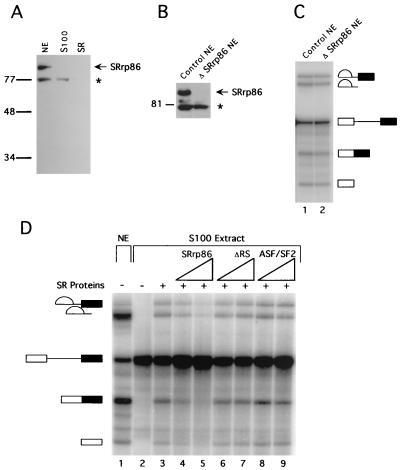
FIG. 2.
SRrp86 inhibits SR proteins but is not required for splicing. (A) Affinity-purified antibodies against SRrp86 were used to probe a Western blot of nuclear extract (NE), S100 extract, and purified SR proteins. The asterisk denotes a 77-kDa protein that cross-reacts with anti-SRrp86 antibodies only under denaturing conditions. (B) Nuclear extracts were immunodepleted of SRrp86 and subjected to Western blot analysis using antibodies against SRrp86. Note that the cross-reactive 77-kDa protein did not coimmunoprecipitate. Molecular masses in kilodaltons are shown to the left of panels A and B. (C) In vitro splicing of an adenovirus-derived substrate was carried out in control extracts or in extracts immunodepleted of SRrp86 (ΔSRrp86 NE). (D) In vitro splicing of the same substrate as in panel C was performed in splicing-deficient S100 extracts in the absence (lane 2) or presence (lane 3) of SR proteins purified from calf thymus (0.5 μg). For comparison, splicing in nuclear extract is shown with the products and intermediates of splicing as indicated. Increasing amounts of SRrp86 (1.5 to 3 μg) inhibited complementation by calf thymus SR proteins (lanes 4 and 5), whereas addition of the ΔRS mutant (1.5 to 3 μg) did not (lanes 6 and 7), nor did the addition of recombinant ASF/SF2 (1.85 to 3.7 μg; lanes 8 and 9).