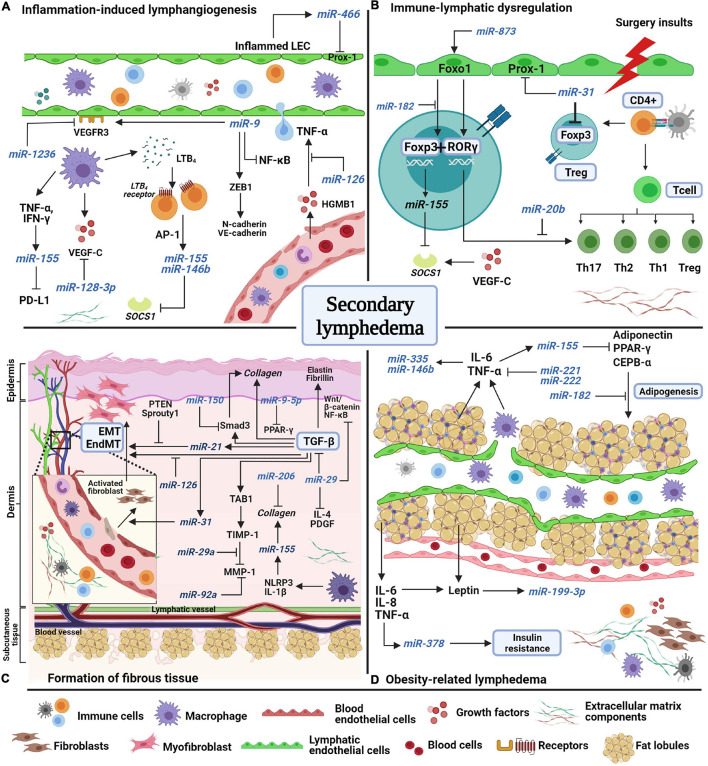
FIGURE 1.
A schematic model of miRNAs’ potential involvement in the pathological features of secondary lymphedema. (A) Inflammation-induced lymphangiogenesis involves the release of inflammatory factors from both lymphatic and blood endothelial cells. Lymphatic markers, VEGF-C and VEGFR-3 are negatively regulated by miR-1236, miR-128-3p, and miR-9. miR-466, miR-155, and miR-146b are expressed during inflammation to induce lymphangiogenesis. (B) Surgery insults to lymphatic vessels and lymph nodes induces abnormal immune regulation as immune cells are trafficked in lymphatic vessels. Due to the disruption of lymph flow, immune cells accumulate in the extracellular matrix (ECM) and start to proliferate and differentiate into regulatory (Treg) and T helper cells, which produce inflammatory factors. Unlike miR-155, miR-31 and miR-182 negatively regulate Foxp3 expression to suppress Treg cell differentiation. Foxo1 induces Foxp3 and Rorγ expression, but the interaction is halted by miR-182 and miR-20b. (C) The formation of fibrous tissue of the skin occurs over time as ECM components increase excessively in the dermis and subcutaneous layers. The main factor of fibrosis, transforming growth factor-beta (TGF-β), mediates collagen fiber production, fibroblast differentiation, and endothelial and epithelial-mesenchymal transformation (EndMT/EMT) by directly regulating pro-fibrotic factors (TAB1, Smad3) and pro-fibrotic miRNAs such as mir-31, miR-21, and miR-155. MiRNAs such as miR-29a, miR-150, miR-9-5p, miR-126, and miR-92 act as anti-fibrotic miRNAs by suppressing the expression of fibrotic factors in multiple signaling pathways. (D) Obesity increases the risk of secondary lymphedema due to excessive accumulation of fat lobules that compress the lymphatic vessels, resulting in disruption of lymph flow. Adipokines and inflammatory factors induce the expression of miR-378, miR-199a-3p, miR-221, miR-222, miR-146b, and miR-335 which facilitate insulin resistance and fat expansion. Several miRNAs act to combat adipogenesis, such as miR-155 and miR-182. AP-1, activating protein-1; CCAAT-enhancer binding protein alpha, CEPB-α; chemokine C-C motif ligand-21, CCL21; cluster of differentiation 4 cells, CD4 +; regulatory T cell, Treg; endothelial-mesenchymal transformation, EndMT; epithelial-mesenchymal transformation, EMT; forkhead box O1, Foxo1; forkhead box P-3, Foxp3; high mobility group box-1 HMGB-1; interferon-gamma, IFN-γ; interleukin, IL; LTB4, leukotriene-B4; matrix metalloproteinase-1, MMP1; nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB; NOD-like receptor protein-3, NLRP3; peroxisome proliferator-activated receptor gamma, PPAR-γ; phosphatase and tensin homolog, PTEN; platelet-derived growth factor, PDGF; programmed death-ligand-1 PD-L1; prospero-homeobox 1 Prox-1; RAR-related orphan receptor gamma, ROR-γ; suppressor of cytokine signaling-1 SOCS1; TGF-beta activated kinase 1, TAB1; T helper cell, Th; TIMP metallopeptidase inhibitor 1, TIMP1; tumor necrosis factor-alpha, TNF-α; vascular endothelial growth factor receptor-3, VEGFR3; zinc finger E-box binding homeobox-1, ZEB1.