Fig. 2 |. Regulation of synaptic transmitter homeostasis and energy metabolites by astrocytes.
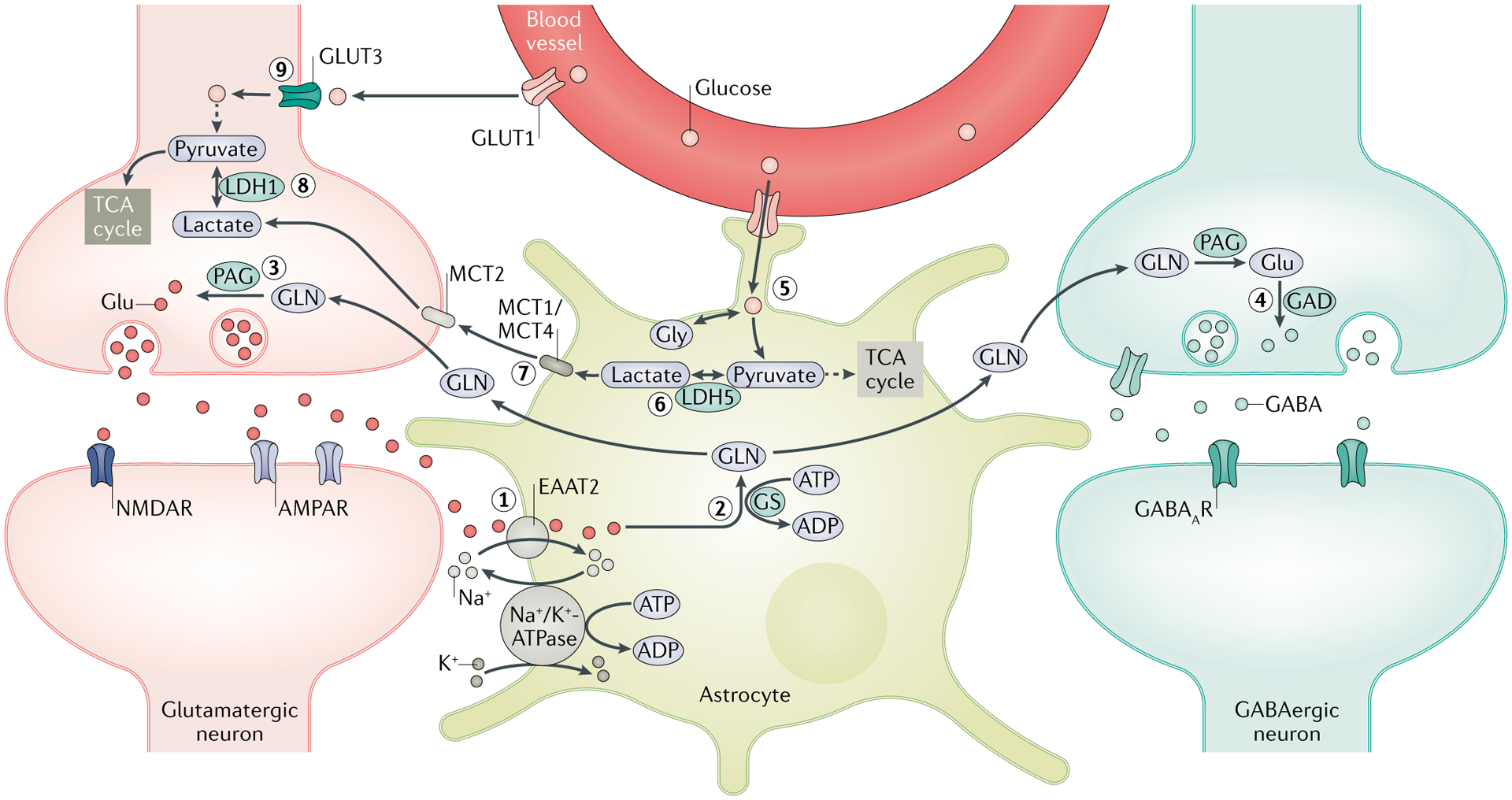
A crucial function of astrocytes is to maintain the optimal level of extracellular glutamate (Glu) through the Glu–glutamine (GLN) cycle. (1) Following synaptic transmission, excess extracellular Glu is absorbed by astrocytes through excitatory amino acid transporter 2 (EAAT2). (2) A large amount of Glu is converted by GLN synthetase (GS) into GLN, which is shuttled into the surrounding neurons. (3) GLN is then metabolized by phosphate-activated glutaminase (PAG) into Glu, which is packaged into synaptic vesicles in the glutamatergic neurons. (4) In GABAergic neurons, Glu is further converted by Glu decarboxylase (GAD) into GABA, which is released in the synapse during inhibitory neurotransmission. Rapid clearance of extracellular Glu following increased activity of synaptic transmission exerts an energetic burden on astrocytes owing to the energy-intensive nature of the Glu–GLN cycle, as both EAAT2 and GS require ATP. (5) The energy deficit that results stimulates the glycolytic oxidation of glucose imported from the blood through glucose transporter 1 (GLUT1) and is enzymatically derived from glycogen stores in astrocytes to generate energy metabolites — lactate and pyruvate. (6) Owing to the limited oxidative capacity of astrocytes, pyruvate is primarily converted into lactate via lactate dehydrogenase 5 (LDH5) and (7) shuttled into the surrounding neurons via monocarboxylate transporters (MCTs). (8) In neurons, LDH1 catalyses the conversion of lactate into pyruvate, which is subsequently used as an energy source through the tricarboxylic acid (TCA) cycle. This pathway is termed the astrocyte neuron lactate shuttle (ANLS) and is crucial in meeting the energy demands of neurons during high synaptic activity, such as during seizures. (9) Glucose is also taken up by neurons from the blood vessels through GLUT3; however, owing to the limited glycolytic capability of neurons and the lack of glycogen stores, neurons derive pyruvate primarily from astrocytes via the ANLS pathway. Disruptions in the expressions and functions of enzymes and transporters implicated in both the Glu–GLN cycle and the ANLS are associated with the development of neuronal hyperexcitability and seizures. AMPAR, AMPA receptor; GABAAR, GABA type A receptor; Gly, glycine; NMDAR, NMDA receptor.
