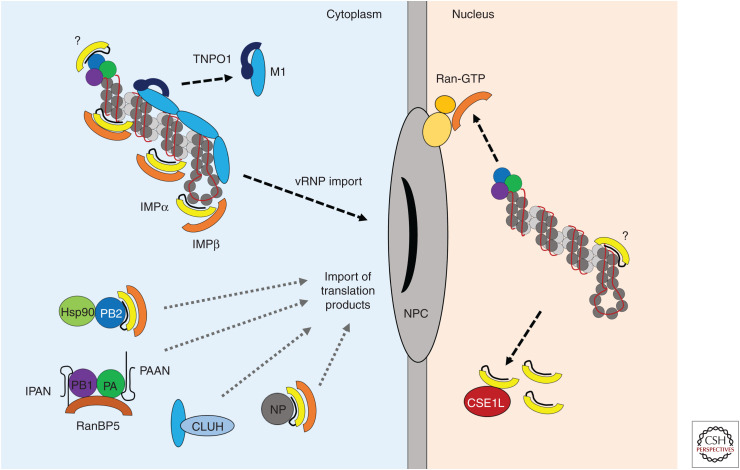
Figure 1.
Nuclear import of viral ribonucleoproteins (vRNPs) and translation products. Following fusion of the viral and endosomal membranes, incoming vRNPs associated with M1 matrix protein enter the cytoplasm. The importin β (IMPβ) family member TNPO1/transportin 1 strips M1 from the vRNPs, thus allowing dissociation of the eight vRNPs from each other and releasing them for interactions with the classical nuclear import machinery. vRNPs interact with importin α (IMPα) adaptor proteins that recognize a nonclassical nuclear localization signal (NLS) at the surface-exposed amino terminus of the nucleoprotein (NP). Direct interaction between IMPα and the polymerase basic 2 (PB2) subunit of the viral RNA–dependent RNA polymerase (RdRp) has also been described, but the biological relevance of this is not known. IMPα is complexed with the IMPβ transport receptor, which mediates translocation into the nucleus by interactions with the nuclear pore complex (NPC). IMPβ is dissociated from the cargo in the nucleus by Ran-GTP associated with the NPC. IMPα is removed separately by CSE1L, although some IMPα may remain associated with the vRNP and support viral replication via unknown mechanisms. The importins subsequently cycle back into the cytoplasm. After primary transcription and translation of viral proteins, NP and RdRp components are transported back into the nucleus to support replication and synthesis of progeny vRNPs. PB2 and NP are imported on their own via classical nuclear import, the former in conjunction with its chaperone Hsp90. PB1 and PA are imported as heterodimers in a nonclassical manner through direct interaction with the β importin RanBP5. PB1 and PA are maintained in a stable conformation and protected against degradation by the nonproteinaceous chaperones long noncoding RNA IPAN (lncRNA-IPAN) and PA-associated noncoding RNA (lncRNA-PAAN), respectively. A subset of newly synthesized M1, required for the vRNP nuclear export complex later in the viral life cycle, is believed to associate with the mitochondrial host protein CLUH for nuclear translocation.