Abstract
Pancreatic cancer is the seventh leading cause of cancer-related deaths worldwide with 432,242 related deaths in 2018. Unlike other cancers, the incidence of pancreatic cancer continues to increase, with little improvement in survival rates. We review the epidemiologic features of pancreatic cancer, covering surveillance and early detection in high-risk persons. We summarize data on worldwide incidence and mortality and analyze the 1975–2016 data from 9 registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results study, on the overall burden of pancreatic cancer as well as age-, sex-, and race-specific incidence, survival rates and trends. It is important to increase our knowledge of the worldwide and regional epidemiologic features of and risk factors for pancreatic cancer, to identify new approaches for prevention, surveillance, and treatment.
Keywords: SEER program, pancreatic adenocarcinoma, PDAC, prevalence
Pancreatic cancer is an increasingly common disease around the world. Most pancreatic cancers (>85%) are adenocarcinoma. Knowledge of both global and local epidemiology and risk factors for pancreatic cancer is essential for contemporary gastroenterology practice.
Global Burden
Globally, pancreatic cancer is the twelfth most common cancer in men, the eleventh most common cancer in women, and the seventh leading cause of cancer-related deaths. Pancreatic cancer is associated with increasing age, and men have slightly higher incidence rates than women.1 Based on the International Agency for Research on Cancer’s (IARC) GLOBOCAN cancer incidence and mortality estimates, there were 458,918 new cases and 432,242 deaths from pancreatic cancer worldwide in 2018, corresponding to 2.5% of all new cancer diagnoses and 4.5% of all cancer deaths, respectively.1 Given the low survival associated with pancreatic cancer, incidence and mortality rates nearly mirror each other. In 2018, the global age-standardized incidence and mortality rates for pancreatic cancer were 4.8 and 4.4 per 100,000 persons, respectively.2 The incidence of pancreatic cancer has increased over recent decades and is projected to continue to rise.3,4 Some proposed reasons for the observed increase in incidence rates include rates of tobacco smoking, obesity, diabetes mellitus, physical inactivity, and consumption of high-calorie/fat diets in certain countries,5–8 combined with improvements in the clinical recognition and diagnosis of pancreatic cancer, and an increasing life expectancy of the global population.9,10 Pancreatic cancer is predicted to soon surpass breast cancer in the European Union to become the third most common cause of cancer-related death,11 as it has already done in the United States (U.S.).4
Variations in Incidence Across Countries
Pancreatic cancer rates are three to four times higher in developed countries, with the world’s highest incidence rates seen in Europe (age-standardized rate (ASR) of 7.7 per 100,000) and North America (ASR of 7.6 per 100,000),1 while the lowest rates of pancreatic cancer are seen in Africa (ASR of 2.2 per 100,000)1 and South-Central Asia (ASR <2.0 per 100,000)9. There is a striking difference in reported pancreatic cancer incidence rates between the country with the highest rate, Hungary (ASR 10.8 per 100,000), and the country with the lowest rate, Guinea (ASR 0.35 per 100,000).12 Countries with higher Human Development Index scores and Gross Domestic Product per capita have notably higher pancreatic cancer case numbers.13 Potential differences among populations to account for these discrepancies in incidence rates include lifestyle habits such as tobacco use and rates of metabolic syndrome/obesity,6–8,14,15 as well as differences in cancer detection, detailed cancer registries and other competing country-specific causes of death. However, large-scale studies describing what factors account for the differences in pancreatic cancer rates across countries are lacking.
Pancreatic Cancer in the United States
Burden and secular trends:
Overall, pancreatic cancer accounts for about 3% of all cancers in the U.S. and about 7% of all cancer deaths. In the U.S., an estimated 56,770 persons (29,940 males and 26,830 females) will be newly diagnosed with pancreatic cancer in 2019, and about 45,750 persons (23,800 males and 21,950 females) will die from their disease. In 2016, pancreatic cancer surpassed breast cancer to be the number three cancer killer in the U.S.4,16 The epidemiology of pancreatic cancer has changed over time in the U.S. For the purpose of this review, we analyzed the most recent data on pancreatic adenocarcinoma, referred to here by the general term pancreatic cancer, from the SEER 9 registries. These registries cover approximately 10% of the U.S. population, where 94,559 cases of invasive pancreatic cancer were diagnosed in SEER 9 registries between 1975 and 2016. Pancreatic adenocarcinoma diagnosis was defined by International Classification of Diseases for Oncology, third edition codes (primary site codes C25.0-C25.3 and C25.7-C25.9) in combination with restricting to microscopically confirmed cases (criteria included positive histology, positive cytology, and positive microscopic confirmation, with method unspecified). The overall incidence rate for pancreatic cancer during 1975–2016 was 9.3 per 100,000 person-years. Pancreatic cancer incidence rates increased from 8.3 per 100,000 person-years in 1975 to 11.0 per 100,000 person-years in 2016. It is projected that within the next one to two decades, pancreatic cancer will surpass colorectal cancer to be the second leading cause of cancer-related deaths and the number one digestive organ cancer killer in the U.S.17
Using the NCI’s Joinpoint program (version 4.1.1; http://surveillance.cancer.gov/joinpoint), joinpoint regression identified two inflection points (in 1984 and 1994). The overall age-standardized incidence rates for pancreatic cancer rose by 0.78% (95% confidence interval [CI], 0.54%−1.02%) annually between 1975 and 2016. Between 1994 and 2016, pancreatic cancer incidence rates have increased at a rate of 1.47% (95% CI, 1.30%−1.63%) per year. In comparing the trend in incidence rates of major U.S. cancer types (prostate, breast, lung and bronchus, colorectal and pancreatic cancer) from 1975–2016, pancreatic cancer is the only malignancy with a constant and steady increase in incidence over that time period (Figure 1). Prostate and breast cancer incidence rates have overall increased but with a more recent declining trend, whereas the incidence rates of lung and colorectal cancer have overall decreased over the last four decades (Figure 2).18
Figure 1:
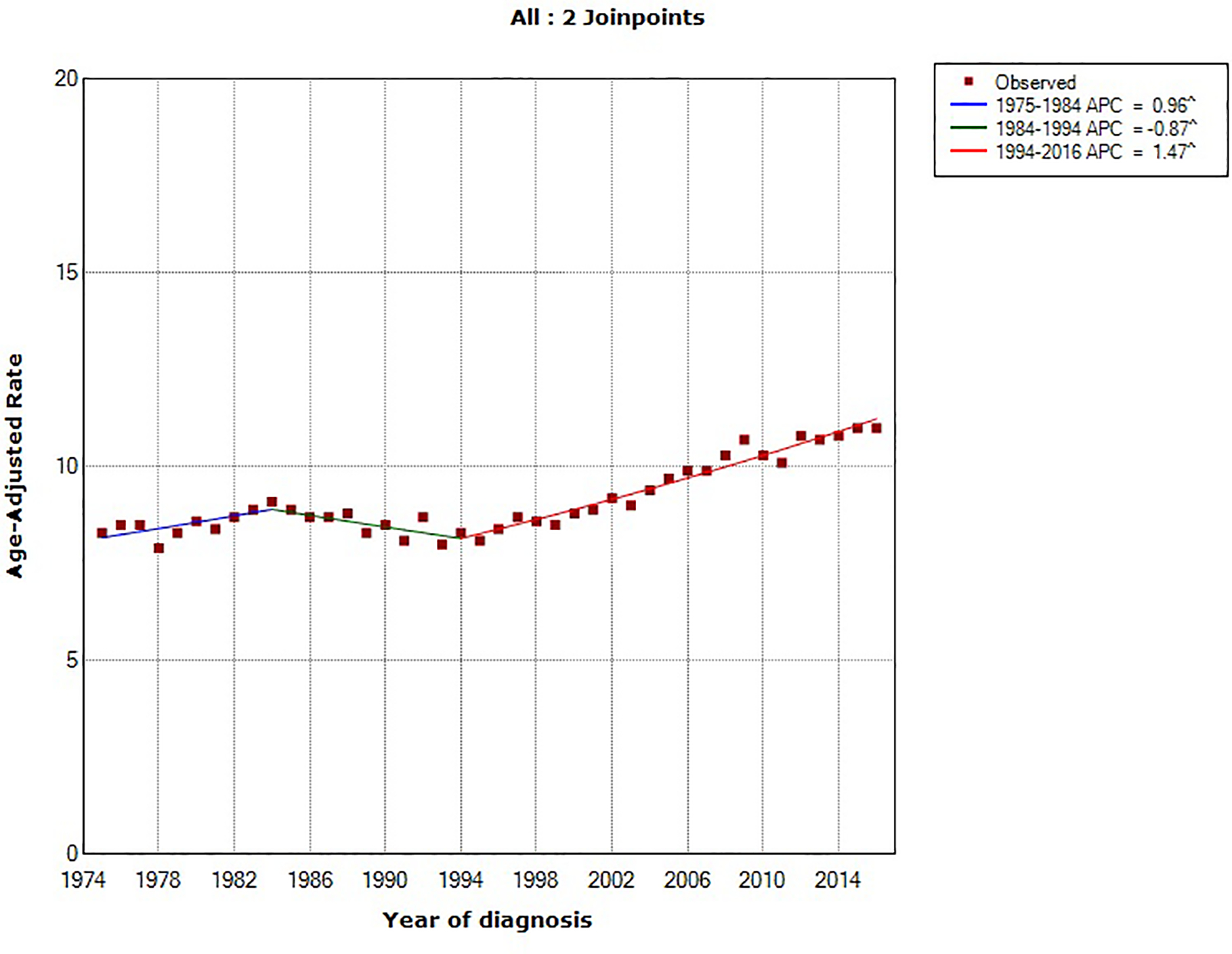
Trends in age-standardized incidence rates for pancreatic cancer in the United States between 1975 and 2016. Source: SEER 9 registries.
Figure 2:
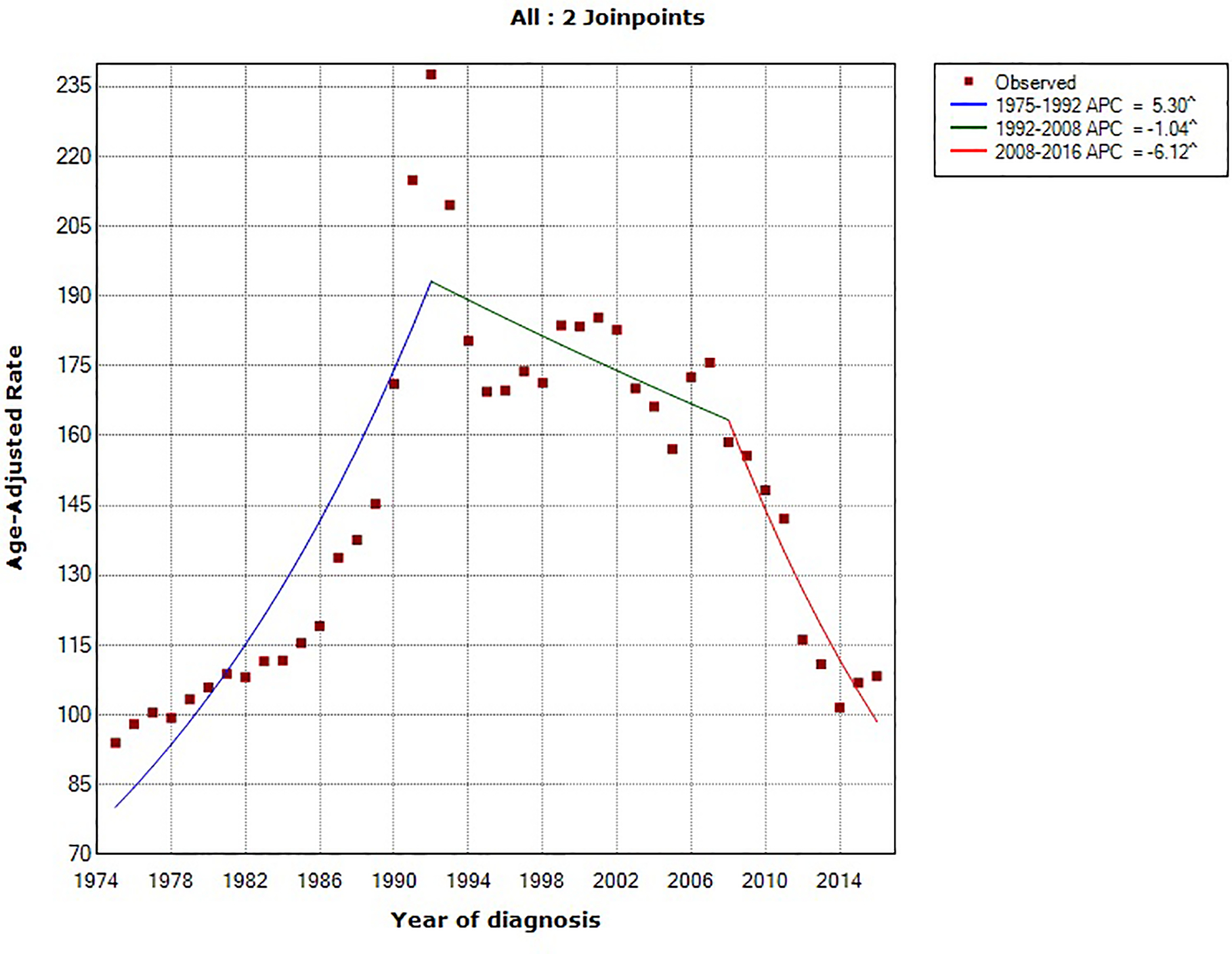
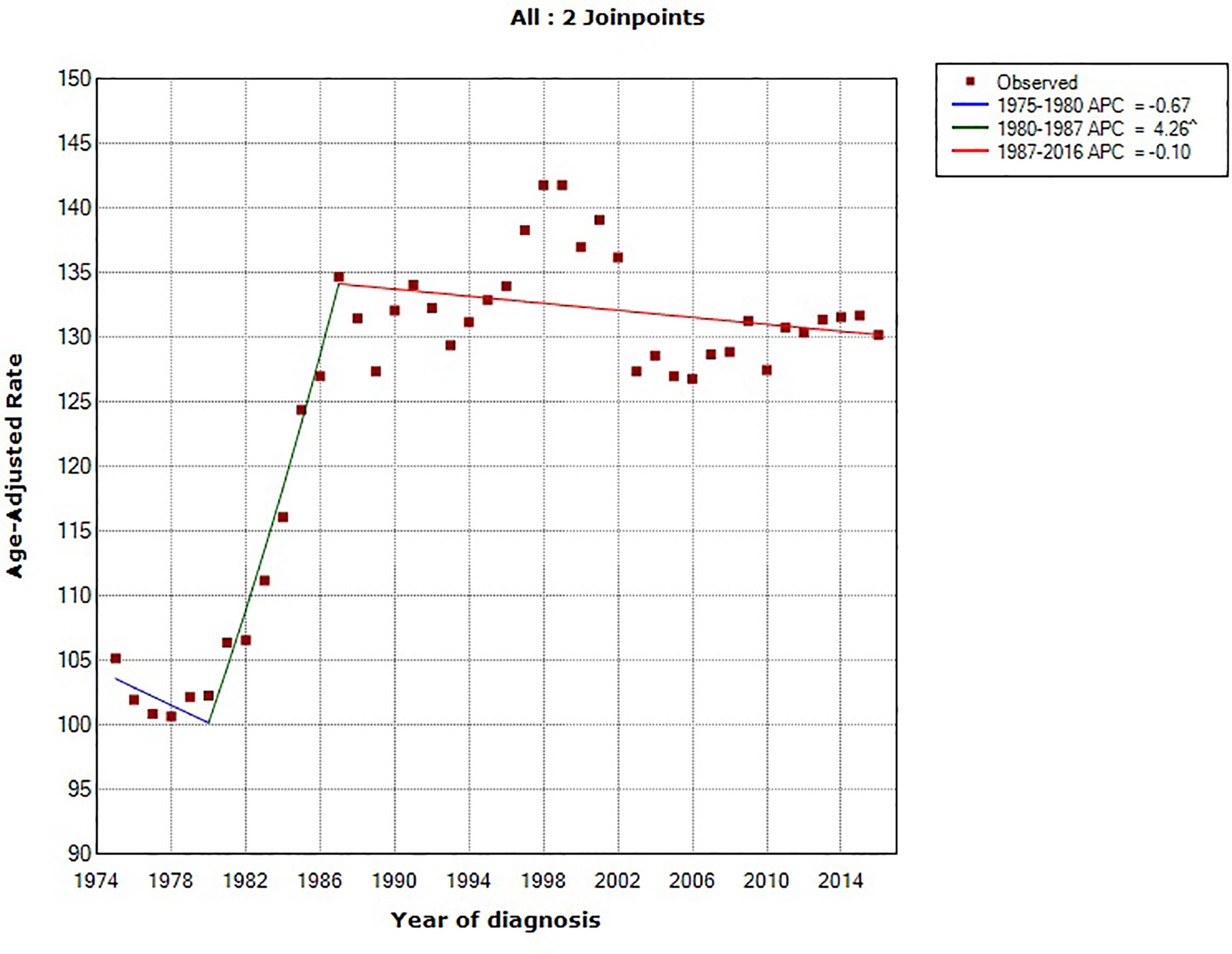
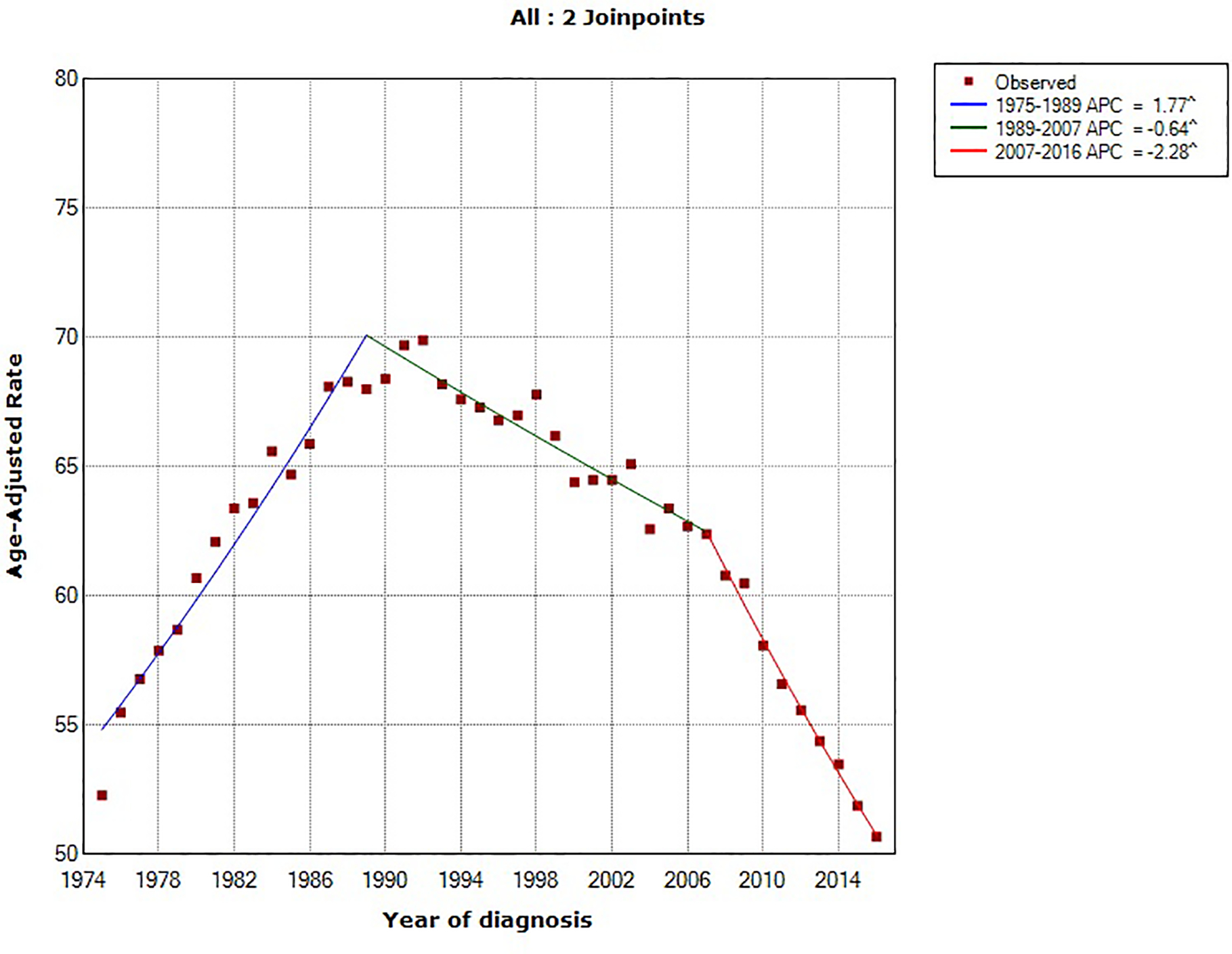
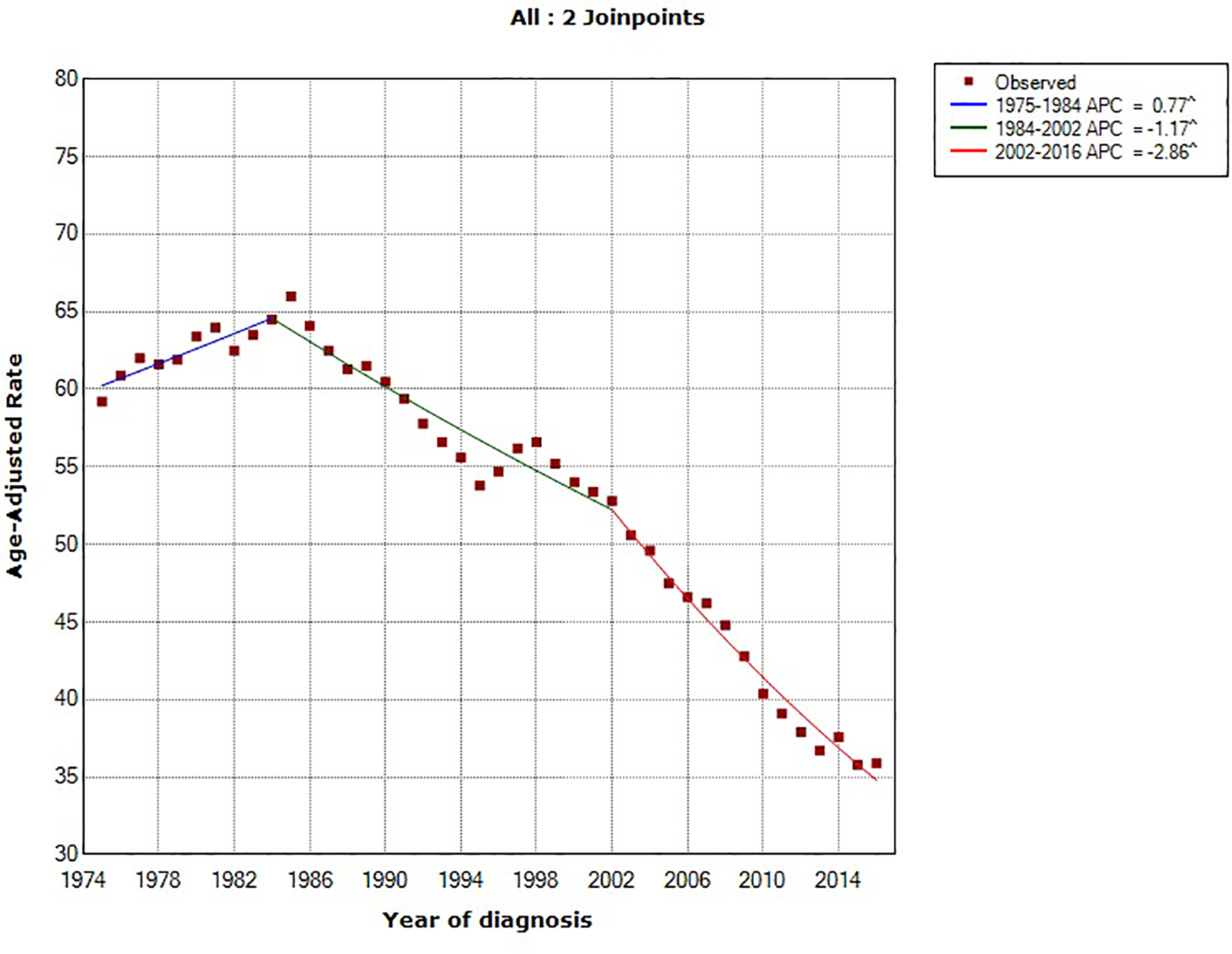
Trends in age-standardized incidence rates for (A) prostate cancer (men only), (B) breast cancer (women only), (C) lung cancer and (D) colorectal cancer in the United States between 1975 and 2016. Source: SEER 9 registries.
Like most cancers, incidence rates for pancreatic cancer in the U.S. increase with increasing age, and the disease is rare among adults under the age of 50. In the SEER 9 registries, only 7.0% (n=6,600) of pancreatic cancer cases diagnosed between 1975 and 2016 were in individuals who were younger than 50 years of age at diagnosis date. Although the absolute incidence rate among individuals under age 50 in the U.S. is still low (1.0/100,000), pancreatic cancer incidence has increased both among individuals aged <50 years (average annual percent change [AAPC] between 1991–2016, 1.44%; 95% CI, 1.00%−1.88%) and ≥50 years (AAPC between 1995–2016, 1.48%; 95% CI, 1.31%−1.66%).
Among all incident pancreatic cancer cases in SEER 9 registries diagnosed between 1975 and 2016, there were almost equal numbers of males (51.7%) and females (48.3%). Between 1975 and 2016, pancreatic cancer incidence rates increased at an average of 0.38% per year in males (AAPC, 0.38%; 95% CI, 0.15%−0.60%) and 1.12% per year in females (AAPC, 1.12%; 95% CI, 0.68%−1.55%).
The incidence rates for pancreatic cancer are higher among Blacks (12.9/100,000 from 1975–2016) than Whites (9.1/100,000 from 1975–2016) in the U.S. However, age-standardized pancreatic cancer incidence rates increased at a faster rate among Whites (AAPC, 0.82%; 95% CI, 0.55%−1.09%) than Blacks (AAPC, 0.33%; 95% CI, 0.14%−0.52%) between 1975 and 2016. In 2016, the age-standard rate for pancreatic cancer was 14.0/100,000 (95% CI, 12.7–15.3) in Blacks and 10.8/100,000 (95% CI, 10.5–11.3) in Whites.
Prognosis for patients with pancreatic cancer:
In the U.S., the overall 5-year relative survival rate for patients diagnosed with pancreatic cancer is among the lowest for all cancer types. However, 5-year relative survival rates have improved modestly from 2.0% (95% CI, 1.7%−2.4%) for individuals diagnosed with pancreatic cancer in the U.S. between 1975 and 1979 to 9.2% (95% CI, 8.7%−9.8%) for those diagnosed between 2007 and 2011. The most important prognostic factor is tumor stage at diagnosis, supporting the critical role for early detection for pancreatic cancer and margin-negative surgical resection. Although the proportion of unstaged cases decreased between 1975 and 2016 (from 11% to 2%), the proportion of pancreatic cancer cases with localized, regional, or distant stage disease (when excluding unstaged cases) remained relatively stable over time (Figure 3). Strikingly, >50% of pancreatic cancer patients in the U.S. are still diagnosed with distant stage disease and only around 10% are diagnosed with localized and therefore resectable disease. For those diagnosed with pancreatic cancer between 2007 and 2011 in the U.S., 5-year relative survival rates decrease from 35.6% in the small proportion of pancreatic cancer patients with localized tumors, to 12.5% and 3.4% for patients with regional and distant stage tumors, respectively (Figure 4). White patients had slightly better stage-adjusted survival rates compared to Black patients (Figure 5). These findings are in keeping with prior U.S. studies in which racial disparities in pancreatic cancer-associated survival rates are seen, with worse survival among Black patients.19,20
Figure 3:
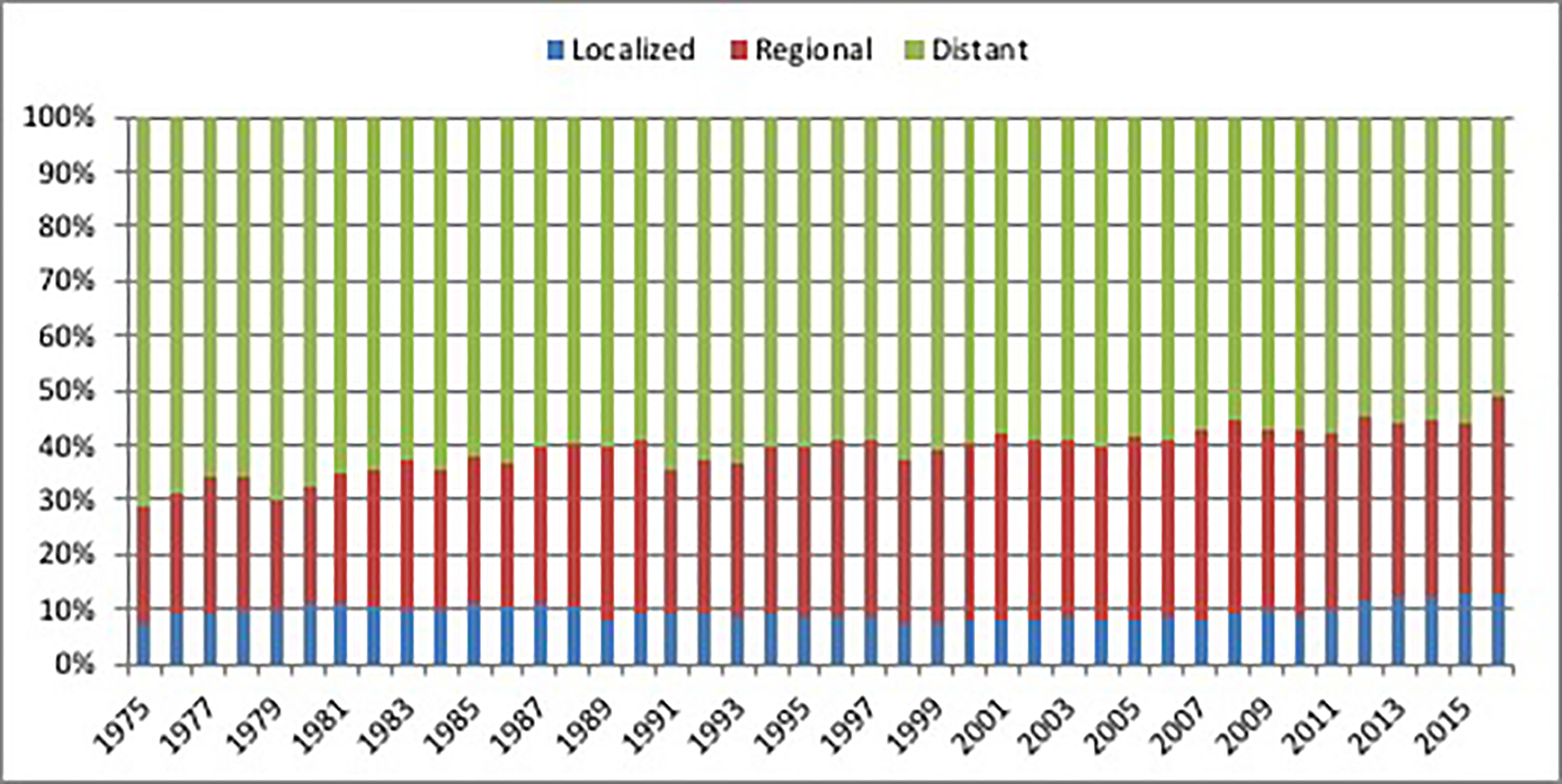
Stage distribution of incident cases of pancreatic cancer in the United States between 1975 and 2016. Source: SEER 9 registries.
Figure 4:
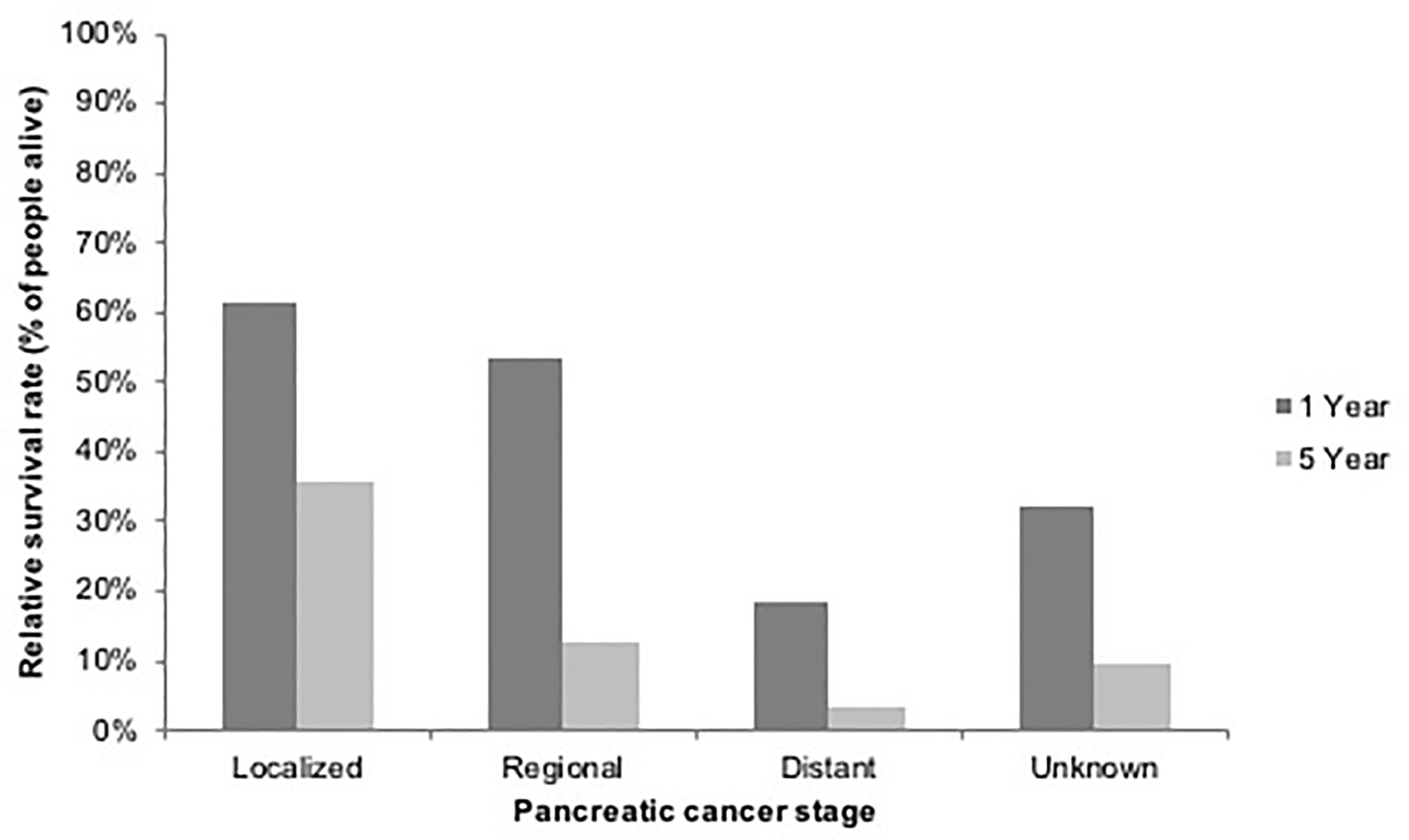
One and five year relative survival rates for patients with pancreatic cancer in the United States between 2007 and 2011 by stage at diagnosis. Source: SEER 9 registries.
Figure 5:
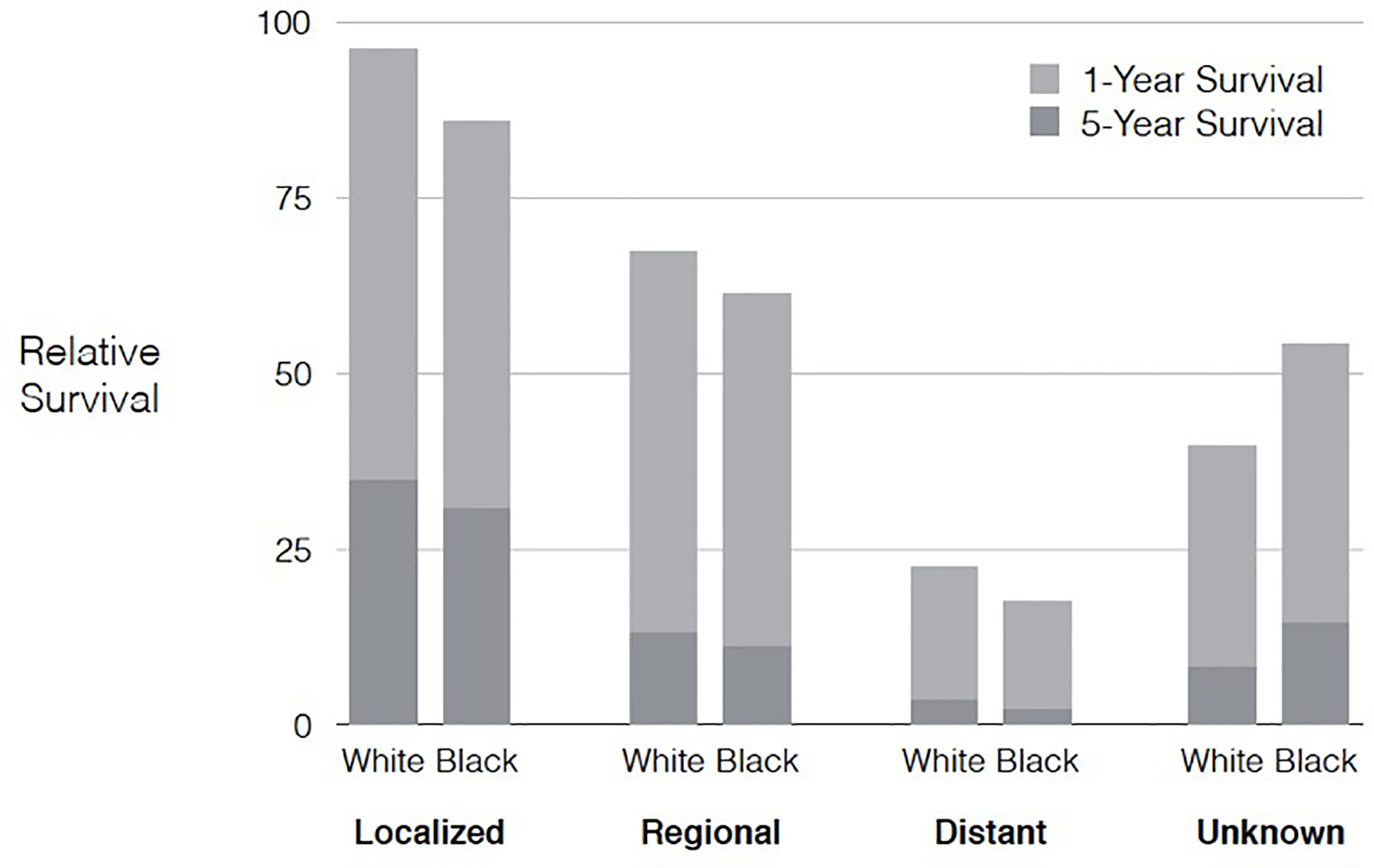
One and five year relative survival rates for patients with pancreatic cancer in the United States between 2007 and 2011 by race and stage at diagnosis. Source: SEER 9 registries.
Risk Factors for Pancreatic Cancer
Important modifiable pancreatic cancer risk factors21 include tobacco smoking5,7,8,22, obesity,7,23–25 physical inactivity23,24,26 and diet type.22,27 Cigarette smoking is the most important environmental factor with a population attributable risk estimated at ~25%.28 Several prospective studies have shown a positive association between red meat and animal fat and risk of pancreatic cancer,29,30 as well as an inverse association between fruits, vegetables, and folate.31,32 In addition, adherence to the Healthy Eating Index 2005 and Mediterranean dietary pattern have been associated with decreased risk of pancreatic cancer.33–35 There is no consistent association between vitamin D supplementation and pancreatic cancer risk.27,36 Other established risk factors for pancreatic cancer that are potentially identifiable in clinical practice21 include inherited or acquired cancer-predisposing genetic mutations/familial syndromes,37–40 mucinous pancreatic cysts,41,42 diabetes mellitus,7,43,44 and chronic pancreatitis.45 There is modest quality evidence for a weak association between certain chronic infections (hepatitis B and C and Helicobacter pylori) and increased pancreatic cancer risk.21,46 Though heavy alcohol consumption is a known risk factor for acute and chronic pancreatitis, the data are insufficient to support its role as an independent risk factor for pancreatic cancer.47,48 In the largest prospective cohort study on the topic, short- or long-term use of aspirin or non-aspirin nonsteroidal anti-inflammatory drugs was not associated with future risk of pancreatic cancer.49 Similarly, statin and metformin use do not seem to decrease the risk of developing pancreatic cancer, though there is some data supporting a survival benefit in persons on these medications after pancreatic cancer diagnosis.50–53
From Epidemiology to Practice
Epidemiological findings should be used to guide both primary prevention through lifestyle modification and secondary prevention through the identification and targeted screening of high risk groups with the goal of cancer prevention and early detection.
Primary cancer prevention:
Building on the epidemiological data summarized above, additional data support that avoiding tobacco use, participating in regular physical activity, and maintaining a healthy weight and diet type can make a significant impact at the population level on reducing pancreatic cancer risk.21 In the Women’s Health Initiative (WHI) dietary modification (DM) randomized controlled trial, a dietary intervention emphasizing a lower-fat dietary pattern that incorporated higher intake of fruits, vegetables, and grains was associated with reduced pancreatic cancer incidence in post-menopausal women who were overweight or obese.54
Surveillance and Risk Stratification
Improving early detection is critical for improving survival in pancreatic cancer. Carbohydrate antigen (CA) 19–9 is the only approved diagnostic marker for pancreatic cancer, however it has limited potential as an early detection tool due to low sensitivity and specificity in early stage disease.55 There are currently no recommended screening programs at the general population level for pancreatic cancer due to its overall low prevalence rate and lack of an easily recognizable and treatable precancerous state.56 However, for populations with an estimated lifetime risk of pancreatic cancer of >5%, some clinical practice guidelines recommend enrollment in surveillance programs.57 Among high-risk patients prospectively enrolled from 1998–2014 in the Johns Hopkins Cancer of the Pancreas Screening program, 3.4% of patients (12 of 354) developed pancreatic cancer over the 16 year follow-up period. Most pancreatic cancers detected during surveillance were early-stage, resectable tumors (71%) with overall 3-year survival rates significantly higher than those seen in sporadic pancreatic cancer cases in the U.S. (57% vs. 8.9%).58 Four major at-risk cohorts have been identified as candidates for targeted screening: patients with germline mutations or family history of pancreatic cancer, mucinous cysts, new onset-diabetes, and patients with pancreatitis.42
Patients with germline mutations or family history of pancreatic cancer.
Consensus recommendations support the enrollment in surveillance programs for those with known genetic syndromes association with pancreatic cancer; those with two relatives with pancreatic cancer, one of whom is a first-degree relative; those with three or more relatives with pancreatic cancer; or those with a history of hereditary pancreatitis (most commonly from PRSS1 gene mutation).59,60 Genetic mutations and syndromes associated with pancreatic cancer include familial pancreatic cancer syndrome, hereditary pancreatitis, familial atypical multiple mole melanoma syndrome, Peutz-Jeghers syndrome, and a subset of Lynch syndrome patients and those with specific germline mutations in pancreatic-cancer predisposing genes (e.g., BRCA1, BRCA2, ATM, PALB2).60 The recommended surveillance program for such patients includes pancreatic imaging with endoscopic ultrasound or magnetic resonance imaging (MRI) annually, beginning at age 50 or 10 years younger than the earliest case of pancreatic cancer in the family.60
Mucinous pancreatic cysts.
Published guidelines utilize mucinous cyst features such as cyst subtype, size, growth rate, presence of mural nodule, enhancing cyst wall, and pancreatic duct dilatation to predict risk of malignant transformation.41,61 This approach does not take into consideration potentially important epidemiological- and patient-specific features. Intraductal papillary mucinous neoplasms (IPMNs), the most common mucinous cyst type, account for ~25% of all cystic pancreatic neoplasms62 and are defined by growth within the pancreatic ducts and production of mucin.61 The lifetime risk of progression of IPMNs to invasive pancreatic adenocarcinoma varies widely, from as low as 6 to as high as 92%,63 highlighting the need for appropriate risk stratification. Though demographic (age, gender), clinical (obstructive jaundice, acute pancreatitis) and radiographic (cyst features, rate of cyst growth) factors have been associated with cancer risk,63–65 most patients who undergo surgery for what are deemed “high-risk” IPMNs do not have high grade dysplasia or malignancy on pathology.66 Unfortunately, limited tools exist to accurately risk stratify the neoplastic potential of IPMNs in clinical practice, and practice guidelines vary by society with deficiencies in each.61,64,66,67
New-onset diabetes.
An estimated 50% of pancreatic cancer patients develop diabetes mellitus prior to their cancer diagnosis, and patients with recently diagnosed or new-onset diabetes have the highest cancer risk due to a paraneoplastic phenomenon.68–71 However, only the vast minority (~1%) of patients with diabetes aged 50 and above will be diagnosed with pancreatic cancer within the 3 years following diabetes diagnosis,68 making the usefulness of new-onset or worsening diabetes in and of itself an inadequate marker for future cancer diagnosis. Therefore, two risk stratification models have emerged in the cohort of patients with new-onset diabetes. The clinical prediction model developed by Boursi et. al from The Health Improvement Network (THIN) database utilizes multiple anthropometric, behavioral, and laboratory variables including age, smoking, indices of diabetes severity, change in body mass index, medications, and alkaline phosphatase level in a defined population of patients with new onset diabetes age 35 and above.72 The model achieves a sensitivity of 45% and specificity of 94% at a 1% probability cutoff (positive predictive value of 2.6%).72 The Enriching New-Onset Diabetes for Pancreatic Cancer (END-PAC) risk stratification model described by Sharma et. al stratifies a population of patients with new-onset diabetes at age 50 and above into high-, intermediate- and low-risk categories for pancreatic cancer based on three factors: change in weight, change in blood glucose, and age at onset of diabetes. Those with high END-PAC scores of ≥3 are categorized as high-risk, and in the absence of an obvious cause for the clinical changes such as steroid use, are recommended to undergo clinical work up for pancreatic cancer.73
Chronic pancreatitis.
Chronic pancreatitis is a fibro-inflammatory pancreatic syndrome with common features of abnormal pancreatic architecture (e.g, glandular fibrosis and atrophy), clinical symptoms (e.g., pain) and organ dysfunction (e.g., exocrine and endocrine insufficiency).74 Patients with chronic pancreatitis are at an increased risk of pancreatic cancer compared to the general population. Approximately 5% of chronic pancreatitis patients will be diagnosed with pancreatic cancer over the two decades following their pancreatitis diagnosis,75,76 believed at least in part a consequence of chronic inflammation and hyperproliferative stellate cells leading to a microenvironment conducive to malignant transformation.77 Among patients with non-hereditary or acquired chronic pancreatitis, the risk of pancreatic cancer is further increased with cigarette smoking and in the setting of new-onset diabetes mellitus.76,78,79
Among patients with hereditary or tropical pancreatitis, the risk of pancreatic cancer is markedly increased compared to the general population and is also higher compared to other groups of chronic pancreatitis patients. In a meta-analysis of 22 studies, the summary relative risks (RRs) for pancreatic cancer were 5.1 (95% CI, 3.5–7.3) for unspecified pancreatitis, 13.3 (95% CI, 6.1–28.9) for chronic pancreatitis, and 69.0 (95% CI, 56.4–84.4) for hereditary pancreatitis.75
Hereditary pancreatitis, an autosomal dominant disease related to mutations in the PRSS1 gene, has classically been reported to carry an incredibly high risk of pancreatic cancer, with a major study of 200 patients showing a cumulative cancer risk of 49% for men and 55% for women by age 75.80 However, in the largest U.S.-specific cohort of hereditary pancreatitis patients (217 patients), this risk was reported as much lower, with a cumulative risk of 7.2% (95% CI 0–15.4) at 70 years of age.81
Despite a well-known increased cancer risk, evidence only supports the benefit of screening for pancreatic cancer among the rare subgroup of patients with hereditary chronic pancreatitis.75 Otherwise, investigating for the presence of pancreatic cancer among chronic pancreatitis patients can be considered in those with new or worsening symptoms such as glucose intolerance, weight loss, malabsorption or pain. Screening for pancreatic cancer among asymptomatic chronic pancreatitis patients or those without new or worsening symptoms is not recommended.
Acute pancreatitis.
Studies suggest risk of pancreatic cancer among acute pancreatitis patients ranges from 0.4–17%82–85 depending on the subpopulation, with highest rates described in those over age 40 with nonalcohol-, nongallstone-related disease. Risk of pancreatic cancer in the first year after acute pancreatitis in patients under age 40 is negligible, while rates rise with increasing age, from 7.7/1000 in the fifth decade of life to 28.7/1000 after age 70.84 Therefore, acute pancreatitis in persons without obvious cause (gallstones, alcohol, etc.), particular in older patients, should prompt suspicion for a possible underlying tumor as the inciting cause. There is also evidence that in the absence of acute pancreatitis occurring because of a malignancy (i.e., cancer diagnosis within a short period after acute pancreatitis episode), there is a two-fold increased risk of pancreatic cancer compared to the general population even up to 5 years after pancreatitis episode.86
Though a handful of higher risk groups have been well-described, additional work is needed to develop improved risk prediction models, noninvasive early detection tests such as circulating biomarkers, and improved imaging techniques better able to identify small/early tumors.
Summary
Here we present an up to date summary of global and national incidence data on pancreatic cancer, a detailed overview of incidence and survival trends over time in the U.S. population, and a description of established as well as suspected risk factors. Our study results highlight that the incidence of pancreatic cancer continues to rise in the U.S., and little improvement in detection at early stage disease or survival has been made over the past several decades. Established risk factors include tobacco smoking, obesity, diet type, those with genetic disorders or family history of pancreatic cancer, mucinous pancreatic cysts, and acute and chronic pancreatitis. By increasing awareness of high risk groups, screening recommendations, and modifiable and nonmodifiable risk factors for pancreatic cancer, clinicians and public health providers can make more informed decisions about patient’s risk, prevention, and need for surveillance.
Acknowledgments
Grant support: The work is supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Abbreviations:
- AAPC
average annual percent change
- ASR
age-standardized rate
- CA
carbohydrate antigen
- CI
confidence interval
- DM
dietary modification
- END-PAC
Enriching New-Onset Diabetes for Pancreatic Cancer
- IARC
International Agency for Research on Cancer
- IPMNs
intraductal papillary mucinous neoplasms
- RR
relative risk
- SEER
Surveillance, Epidemiology, and End Results
- THIN
The Health Improvement Network
- U.S.
United States
- WHI
Women’s Health Initiative
Footnotes
Conflicts of interest: No relevant conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941–1953. [DOI] [PubMed] [Google Scholar]
- 3.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699–708. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 1996;156:2255–2260. [PubMed] [Google Scholar]
- 6.Doll R, Peto R, Boreham J, et al. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer 2005;92:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson SC, Permert J, Håkansson N, et al. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer 2005;93:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 2009;170:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathers CD, Fat DM, Inoue M, et al. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol Stockh Swed 2016;55:1158–1160. [DOI] [PubMed] [Google Scholar]
- 12.Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong MCS, Jiang JY, Liang M, et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep 2017;7:3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arslan AA. Anthropometric Measures, Body Mass Index, and Pancreatic Cancer: A Pooled Analysis From the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 17.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2018 Sub (1975–2016), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2019, based on the November 2018 submission. [Google Scholar]
- 19.Khawja SN, Mohammed S, Silberfein EJ, et al. Pancreatic cancer disparities in African Americans. Pancreas 2015;44:522–527. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970–2009. J Natl Cancer Inst 2013;105:1694–1700. [DOI] [PubMed] [Google Scholar]
- 21.Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett 2016;381:269–277. [DOI] [PubMed] [Google Scholar]
- 22.Jarosz M, Sekula W, Rychlik E. Influence of diet and tobacco smoking on pancreatic cancer incidence in poland in 1960–2008. Gastroenterol Res Pract 2012;2012:682156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 24.Patel AV, Rodriguez C, Bernstein L, et al. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2005;14:459–466. [DOI] [PubMed] [Google Scholar]
- 25.Zhou B, Wu D, Liu H, et al. Obesity and pancreatic cancer: An update of epidemiological evidence and molecular mechanisms. Pancreatol Off J Int Assoc Pancreatol IAP Al August 2019. [DOI] [PubMed] [Google Scholar]
- 26.Berrington de González A, Spencer EA, Bueno-de-Mesquita HB, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2006;15:879–885. [DOI] [PubMed] [Google Scholar]
- 27.Pericleous M, Rossi RE, Mandair D, et al. Nutrition and pancreatic cancer. Anticancer Res 2014;34:9–21. [PubMed] [Google Scholar]
- 28.Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol 2004;34:238–244. [DOI] [PubMed] [Google Scholar]
- 29.Taunk P, Hecht E, Stolzenberg-Solomon R. Are meat and heme iron intake associated with pancreatic cancer? Results from the NIH-AARP diet and health cohort. Int J Cancer 2016;138:2172–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. Br J Cancer 2012;106:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsamarrai A, Das SLM, Windsor JA, et al. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2014;12:1635–1644.e5; quiz e103. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q-J, Wu L, Zheng L-Q, et al. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 2016;25:196–205. [DOI] [PubMed] [Google Scholar]
- 33.Arem H, Reedy J, Sampson J, et al. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J Natl Cancer Inst 2013;105:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosetti C, Bravi F, Turati F, et al. Nutrient-based dietary patterns and pancreatic cancer risk. Ann Epidemiol 2013;23:124–128. [DOI] [PubMed] [Google Scholar]
- 35.Jiao L, Mitrou PN, Reedy J, et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med 2009;169:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S-L, Zhao Y-P, Dai M-H, et al. Vitamin D status and the risk of pancreatic cancer: a meta-analysis. Chin Med J (Engl) 2013;126:3356–3359. [PubMed] [Google Scholar]
- 37.Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer 2009;8:109–117. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Chen S, Brune KA, et al. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol Off J Am Soc Clin Oncol 2007;25:1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Brune KA, Visvanathan K, et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 2009;18:2829–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon S, Das S, Brand R, et al. Inherited pancreatic cancer syndromes. Cancer J Sudbury Mass 2012;18:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2013;11:913–921; quiz e59–60. [DOI] [PubMed] [Google Scholar]
- 42.Singhi AD, Koay EJ, Chari ST, et al. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019;156:2024–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer Oxf Engl 1990 2011;47:1928–1937. [DOI] [PubMed] [Google Scholar]
- 45.Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 2002;51: 849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatol Baltim Md 2009;49:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velema JP, Walker AM, Gold EB. Alcohol and pancreatic cancer. Insufficient epidemiologic evidence for a causal relationship. Epidemiol Rev 1986;8:28–41. [DOI] [PubMed] [Google Scholar]
- 48.Ye W, Lagergren J, Weiderpass E, et al. Alcohol abuse and the risk of pancreatic cancer. Gut 2002;51:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalaf N, Yuan C, Hamada T, et al. Regular Use of Aspirin or Non-Aspirin Nonsteroidal Anti-Inflammatory Drugs Is Not Associated With Risk of Incident Pancreatic Cancer in Two Large Cohort Studies. Gastroenterology 2018;154:1380–1390.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HS, Lee SH, Lee HJ, et al. Statin Use and Its Impact on Survival in Pancreatic Cancer Patients. Medicine (Baltimore) 2016;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jian-Yu E null, Graber JM, Lu S-E, et al. Effect of Metformin and Statin Use on Survival in Pancreatic Cancer Patients: a Systematic Literature Review and Meta-analysis. Curr Med Chem 2018;25:2595–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada T, Khalaf N, Yuan C, et al. Prediagnosis Use of Statins Associates With Increased Survival Times of Patients With Pancreatic Cancer. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2018;16:1300–1306.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamada T, Khalaf N, Yuan C, et al. Statin use and pancreatic cancer risk in two prospective cohort studies. J Gastroenterol 2018;53:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiao L, Chen L, White DL, et al. Low-fat Dietary Pattern and Pancreatic Cancer Risk in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3:105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.US Preventive Services Task Force. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/pancreatic-cancer-screening. Accessed May 5, 2019.
- 57.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Canto MI, Almario JA, Schulick RD, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018;155:740–751.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood LD, Yurgelun MB, Goggins MG. Genetics of Familial and Sporadic Pancreatic Cancer. Gastroenterology 2019;156:2041–2055. [DOI] [PubMed] [Google Scholar]
- 60.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–262; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elta GH, Enestvedt BK, Sauer BG, et al. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol 2018;113:464–479. [DOI] [PubMed] [Google Scholar]
- 62.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch Int J Pathol 2004;445:168–178. [DOI] [PubMed] [Google Scholar]
- 63.Machado NO, Al Qadhi H, Al Wahibi K. Intraductal Papillary Mucinous Neoplasm of Pancreas. North Am J Med Sci 2015;7:160–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2017;17:738–753. [DOI] [PubMed] [Google Scholar]
- 65.Marchegiani G, Fernández-del Castillo C. Is it safe to follow side branch IPMNs? Adv Surg 2014;48:13–25. [DOI] [PubMed] [Google Scholar]
- 66.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824–848.e22. [DOI] [PubMed] [Google Scholar]
- 67.Xu M-M, Yin S, Siddiqui AA, et al. Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms. Medicine (Baltimore) 2017;96:e7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aggarwal G, Rabe KG, Petersen GM, et al. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatol Off J Int Assoc Pancreatol IAP Al 2012;12:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boursi B, Finkelman B, Giantonio BJ, et al. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients With New-Onset Diabetes. Gastroenterology 2017;152:840–850.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018;155:730–739.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatol Off J Int Assoc Pancreatol IAP Al 2016;16:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010;24:349–358. [DOI] [PubMed] [Google Scholar]
- 76.Hart PA, Conwell DL. Chronic Pancreatitis: Managing a Difficult Disease. Am J Gastroenterol November 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Algül H, Treiber M, Lesina M, et al. Mechanisms of disease: chronic inflammation and cancer in the pancreas--a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol 2007;4:454–462. [DOI] [PubMed] [Google Scholar]
- 78.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993;328:1433–1437. [DOI] [PubMed] [Google Scholar]
- 79.Liao K-F, Lai S-W, Li C-I, et al. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol 2012;27:709–713. [DOI] [PubMed] [Google Scholar]
- 80.Rebours V, Boutron-Ruault M-C, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series. Am J Gastroenterol 2008;103:111–119. [DOI] [PubMed] [Google Scholar]
- 81.Shelton CA, Umapathy C, Stello K, et al. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol 2018;113:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rijkers AP, Bakker OJ, Ahmed Ali U, et al. Risk of Pancreatic Cancer After a Primary Episode of Acute Pancreatitis. Pancreas 2017;46:1018–1022. [DOI] [PubMed] [Google Scholar]
- 83.Tummala P, Tariq SH, Chibnall JT, et al. Clinical predictors of pancreatic carcinoma causing acute pancreatitis. Pancreas 2013;42:108–113. [DOI] [PubMed] [Google Scholar]
- 84.Munigala S, Kanwal F, Xian H, et al. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2014;12:1143–1150.e1. [DOI] [PubMed] [Google Scholar]
- 85.Chung S-D, Chen K-Y, Xirasagar S, et al. More than 9-times increased risk for pancreatic cancer among patients with acute pancreatitis in Chinese population. Pancreas 2012;41:142–146. [DOI] [PubMed] [Google Scholar]
- 86.Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, et al. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology 2018;154:1729–1736. [DOI] [PubMed] [Google Scholar]


