Figure 2. Examples of nanomaterial-based strategies used to combat bacterial infections.
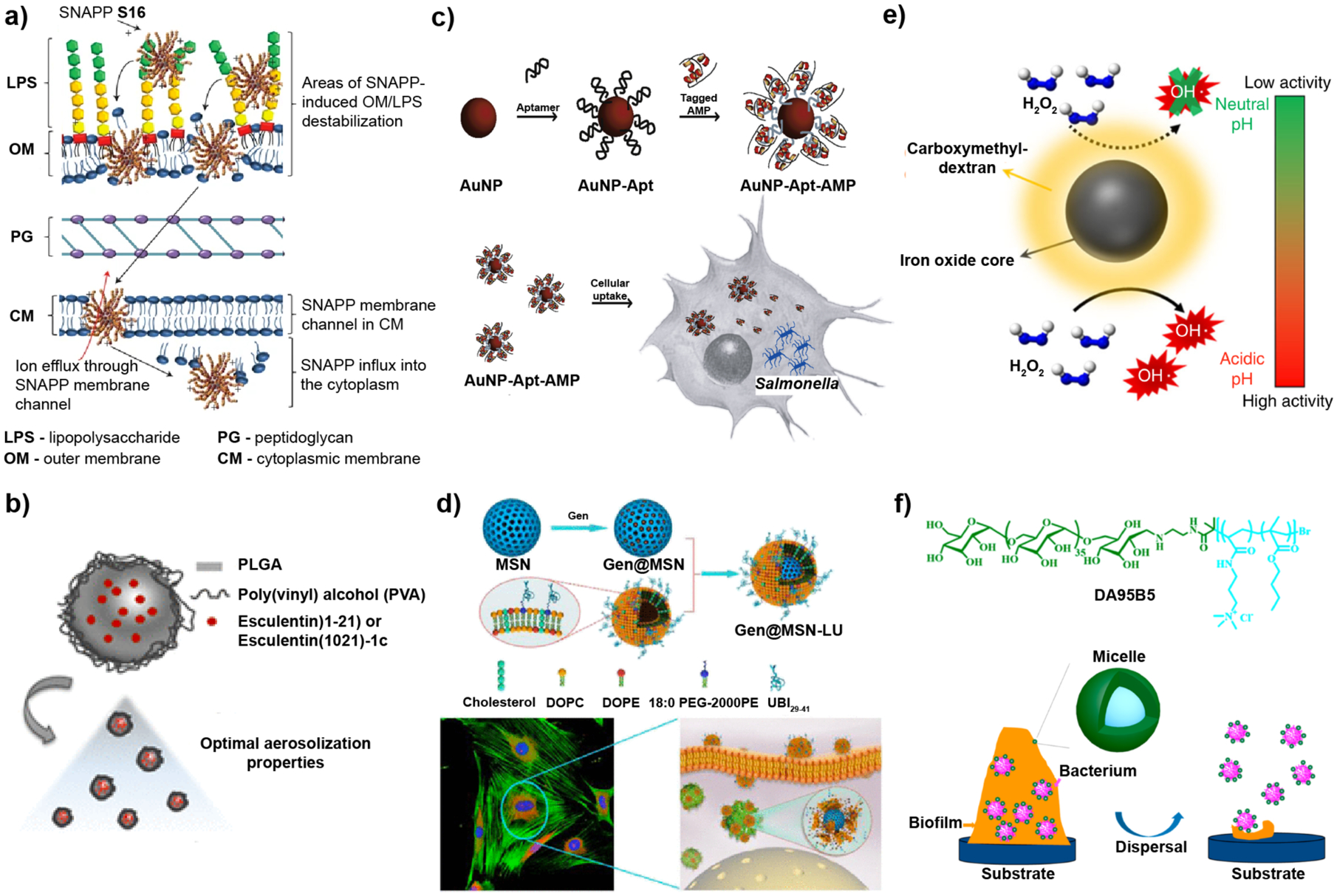
a-b| Planktonic bacterial infections. c-d| Intracellular infections. e-f| Biofilm infections. a| Structurally engineered AMPs, SNAPPs, exhibited promising antimicrobial activity in vitro and in vivo. SNAPPs interacts with the outer membrane, peptidoglycan and cytoplasmic membrane layers of bacteria through electrostatic interactions, ultimately leading to cell lysis. b| Intratracheal administration of antimicrobial esculentin-1a formulated to be delivered to the lungs using PLGA NPs reduced P. aeruginosa lung infection in a mouse model. c| Histidine-aptamer-conjugated gold nanoparticles loaded with His-tagged AMPs were effective for treatment of Salmonella enterica-infected mammalian cells. d| Gentamicin-loaded mesoporous silica nanoparticles with a bacterial toxin-responsive lipid bilayer surface shell and bacteria-targeting peptide UBI29–41, allowed targeted release of antibiotic for killing of intracellular S. aureus. e| A carboxymethyl-dextran-coated iron oxide nanoparticle, ferumoxytol, catalyzed ROS production of H2O2 in a pH-dependent manner as a treatment against oral biofilms. f| Dextran (green) and poly(AMPTMA-co-BMA) (light blue) form a micelle with a bactericidal core and non-fouling dextran shell used to treat wound biofilms. Electrostatic interaction of the NPs with the biofilm weakens bacterial attachment while gradually dispersing EPS matrix. Image in part a reproduced, with permission, from REF 63 © (2016) Macmillan Publishers Limited, part of Springer Nature. Image in part b reproduced, with permission, from REF 95 © (2019) American Chemical Society. Image in part c reproduced, with permission, from REF 96 © (2016) Elsevier Ltd. Image in parts d and f reproduced, with permission, from REF 97 and 98, respectively © (2018) American Chemical Society. Image in part e reproduced, with permission, from REF. 99 © (2018) Nature Communications. All rights reserved.
