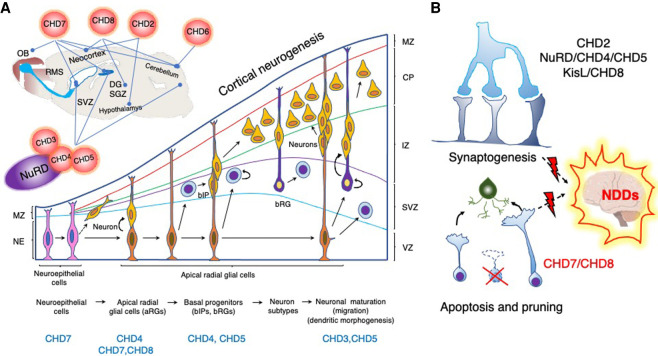
Figure 5.
CHDs regulate different aspects of neurodevelopment. (A, top) CHD enzymes are vital for the development and functionality of different parts of the brain: the cerebral neocortex (CHD2, CHD3/4/5/NuRD, CHD7, and CHD8), cerebellum (CHD4/NuRD, CHD6, CHD7, and CHD8), hypothalamus (CHD2), thalamus (CHD8), and hippocampus (CHD4/5/NuRD, CHD7, and CHD8). They regulate neural stem cell niches in the cerebrum (subventricular zone [SVZ]) and hippocampus (subgranular zone of the dentate gyrus [SGZ/DG]) and are essential for proper neuronal differentiation and migration. CHD7 is important for the development of the inner ear and olfactory system and regulates migration of the neuroblasts that emerge from the neural stem cells in the SVZ and proceed through the rostral migratory stream (RMS) to the olfactory bulb (OB). The cartoon represents the sagittal section of the rodent brain and highlights CHD functions in the respective brain regions (lines). (Bottom) CHDs are required at various stages of cortical neurogenesis. During the early stages of cortical neurogenesis, symmetrical division of the neuroepithelial cells (NEs) in the ventricular zone (VZ) expands their pool, whereas asymmetrical division gives rise to apical neural precursor cells (NPCs), such as apical radial glial cells (aRGs), and pioneer neurons. Apical progenitor cells give rise to neurons through basal intermediate precursors (bIP) and radial glial cells (bRG) that reside in the subventricular zone (SVZ). Neurons use aRG and bRG fibers to migrate to the specified layer in the cortical plate (CP), where they establish synaptic connections with subplate layer neurons during maturation. CHD remodelers involved at distinct stages are highlighted bellow. (MZ) Marginal zone, (IZ) intermediate zone. Cortical neurogenesis cartoon representation adapted with permission from Sokpor et al. (2018). (B, top) Synaptogenesis between neurons initiates during embryogenesis, but synaptic plasticity proceeds throughout life. Synaptic plasticity is the property of synapses to strengthen or weaken in response to changes in both the amplitude and the temporal dynamics of neuronal activity. Sensory inputs and intrinsic brain activity can effect long-term changes in synaptic efficacy and eventually increase or decrease neuronal connectivity by modulating the number of synapses (Bourgeron 2015). Some CHD enzymes regulate neuronal connectivity (CHD4, CHD5, and CHD8) and excitability (CHD2 and CHD8), whereas others regulate synaptic vesicle recycling (KisL) and expression of genes essential for synaptic homeostasis and plasticity (CHD8). (Bottom) Apoptosis and pruning are required for the maintenance and refinement of the neural circuitries, and CHD7/8 inhibition of P53-mediated apoptosis plays an active role in this process. Dysregulation of CHD enzyme function affects synaptogenesis and apoptosis and leads to aberrant neurogenesis and connectivity, which results in the emergence of diverse neurodevelopmental disorders (NDDs).