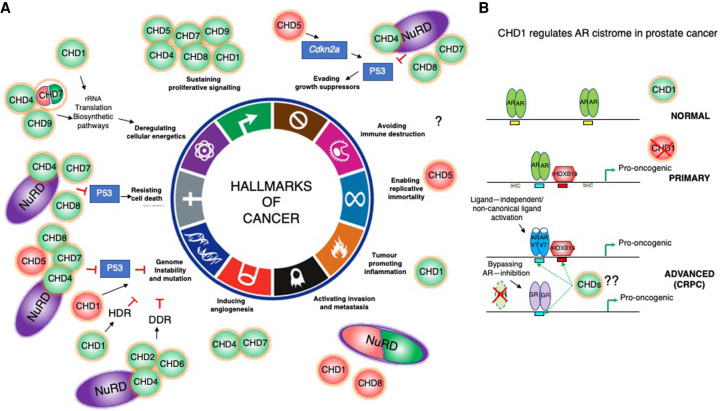
Figure 6.
The role of CHDs in tumorigenesis. (A) CHD enzymes are context-dependent tumor suppressors (TS; red) and oncogenes (green) that contribute to acquisition of different hallmarks of cancer. They can drive tumorigenesis by directly promoting transcription of oncogenes and repression of TS genes (CDKN2A and TP53; blue rectangles). Some enzymes can exert both oncogenic and TS function (CHD1, CHD4/NuRD, CHD7, and CHD8). For simplicity, not all players that are mentioned in the text are indicated in the cartoon. CHD functions in human malignancies are still largely unexplored, and it is likely that all members are dysregulated and mutated in diverse cancers and contribute to different aspects of tumorigenesis. Cartoon representation adapted from Hanahan and Weinberg (2011) with permission from Elsevier. (B, top) CHD1 nucleosome remodeling activity restricts binding of AR (green ovals) to prostetic lineage enhancers (yellow rectangles) in normal prostates. (Middle) During prostate tumorigenesis, in primary tumors, CHD1 loss leads to AR cistrome redistribution to a different subset of enhancers (blue rectangles), with HOXB13 (red hexagon) binding motifs (red squares) to drive expression of pro-oncogenic gene networks. (Bottom) More advanced, castration-resistant prostate cancers (CRPCs) are characterized by amplifications or mutations in the AR ligand-binding domain (LBD; AR-V7 as an example; blue ovals) (Jeselsohn et al. 2015; Watson et al. 2015). The LBD mutations allow promiscuous activation by noncanonical ligands (adrenal androgens, estrogen, progesterone, and glucocorticoids) to secure sustained expression of a subset of critical AR target genes (Watson et al. 2015). Additionally, antiandrogen-resistant prostate cancers can fully bypass the requirement for AR signaling by activating GR-mediated (violet ovals) transcriptional regulation of the AR cistrome (Arora et al. 2013). The role of other CHDs in the regulation of alternative transcriptional pathways that sustain expression of the AR-driven oncogenic gene networks in CRPC prostate cancers is unknown.