Figure 1.
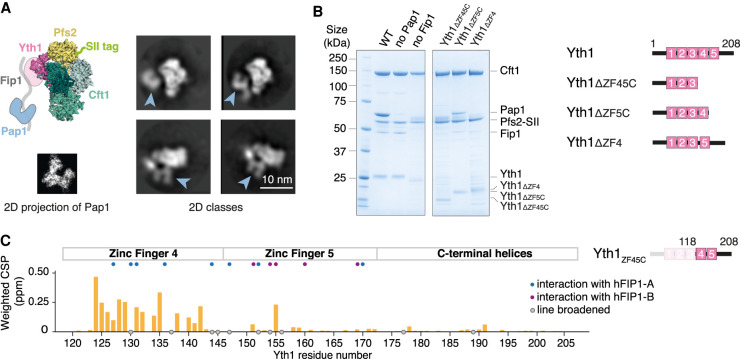
Fip1 binds Yth1 and tethers Pap1 to the polymerase module. (A) Cartoon representation (top left) and selected 2D class averages from cryoEM (right) of the polymerase module. The 2D averages show a central structure corresponding to the Cft1-Pfs2-Yth1 subunits, and an additional horseshoe-shaped density (blue arrowheads). (Bottom left) Similarity to a 2D projection of the crystal structure of Pap1 (PDB 3C66) suggests that the horseshoe-shaped density is Pap1. (SII) StrepII. (B) SDS-PAGE of pull-down assays using StrepII-tagged (SII) Pfs2 reveals interactions within the polymerase module. (Right) Domain diagrams of Yth1 indicate the constructs used. Fip1 is required for Pap1 interaction. When the interaction between Yth1 and Fip1 is compromised, Fip1 is not pulled down and therefore Pap1 is also absent. Yth1 subunits in the WT and “no Pap1” complexes contain a His tag, whereas Yth1 in the “no Fip1” and Yth1 truncation complexes do not contain a His tag. With Yth1ΔZF4, protein degradation (potentially from Pfs2) is evident. (C) Histogram showing chemical shift perturbations (CSPs) in Yth1ZF45C (residues 118–208) spectra upon Fip1226 binding. Yth1ZF45C and Fip1226 were mixed in an equimolar ratio to a final concentration of 75 µM. Gray circles indicate peaks that showed exchange broadening upon Fip1 binding. Most of the peaks that are perturbed are in zinc finger 4. Homologous residues contributing >50 Å2 buried surface area in the human FIP1-CPSF30 structure (Hamilton and Tong 2020) are highlighted with blue or purple circles. In that structure, two hFIP1 molecules (hFIP1-A and hFIP1-B) are bound to one CPSF30.