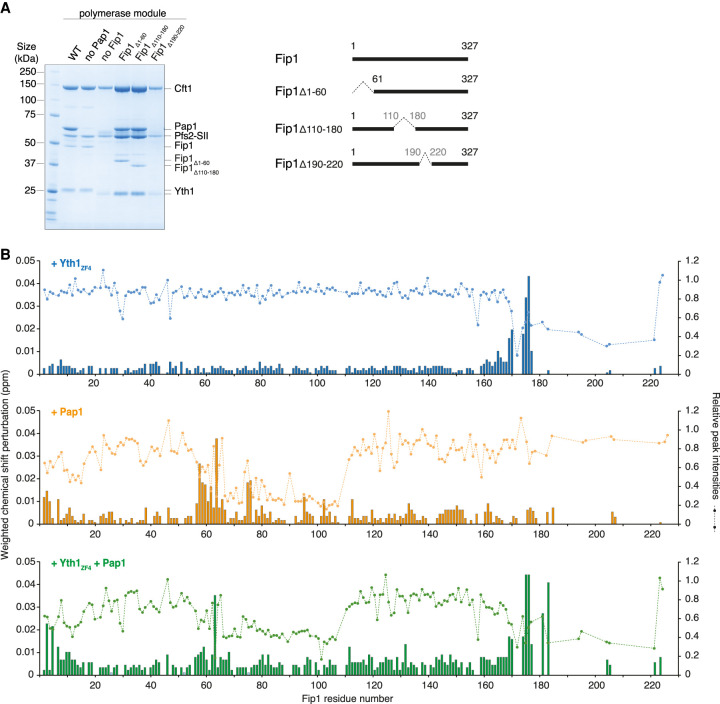
Figure 3.
Fip1226 contains bipartite binding sites for Yth1 and Pap1. (A) SDS-PAGE of pull-down assays using StrepII-tagged (SII) Pfs2. Residues 190–220 on Fip1 are essential for reconstitution of the polymerase module. Yth1 is 6His-tagged for wild-type (WT) and “no Pap1” samples, but untagged in all other samples. Diagrams of full-length and truncated Fip1 are shown at the right. The first three lanes (WT, no Pap1 and no Fip1) are reproduced from Figure 1B. (B) Chemical shift perturbations of Fip1226 upon binding of Yth1ZF4 (blue, top), Pap1 (orange, middle), and Yth1ZF4 and Pap1 together (green, bottom). Chemical shift perturbations are shown as histograms. The dotted line indicates the relative peak intensities compared with free Fip1226 in the 1H,15N 2D HSQC spectra. Experiments were performed at 150 mM NaCl to minimize potential nonspecific binding.