Table 3.
Summary of included studies.
| References |
Location Design |
Intervention | Other treatments | Other medications | Outcomes | Significant findings | Adverse events |
|---|---|---|---|---|---|---|---|
| Jiang et al. (9) | United Kingdom Case series | Ilgwanjeon (一貫煎) Yugmijihwangwon ( 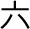 味地黃元) 味地黃元)Bojungikgitang (補中益氣湯) Sammyohwan (三妙丸) Jingansikpungtang (鎭肝熄風湯) |
Scalp acupuncture Body acupuncture Electro acupuncture | No mention | Four class recovery criteria of the effects of TCM treatment | Improved the symptoms of MS, regulated immune system and promoted nerve regeneration if used over a long period of time, but herbal medicines alone were not powerful enough to treat MS effectively -TER: 90% (excellent results rate of 25%) |
No mention |
| He et al. (16) | China Case series | Hojamhwan (虎潛丸) |
Acupuncture | No mention | Principles for guiding clinical study on the treatment of stroke with new Chinese drugs | -TER: 81.82% | No mention |
| Kang et al. (17) | Korea Case report | Dossibojungikgitang (陶氏補中益氣湯) Gamiondamtang (加味溫膽湯) Hyangsayukgunjatang (香砂六君子湯) Gamisodamjeseuptang (加味消痰除濕湯) Yeongseonjetongeumgagam (靈仙除痛飮加減) |
Acupuncture | No mention | Clinical symptoms SAEST | Improved muscular strength & numbness of the limb, dyspepsia, anxiety and insomnia, dysuria Declined back pain - SAEST (Rt./Lt.): S/J: 1/2 → 4/4 F/J: 1/2 → 4/4 H/J: 1/2 → 3/4 K/J: 1/3 → 4/4 Foot,Toe: 2/2 → 3/4 Total: 0 → 3 |
No mention |
| Hwang et al. (18) | Korea Case report | Cheongsimyeonjaeumgami (淸心  子飮加味) 子飮加味) |
Acupuncture Moxibustion | No mention | Clinical symptoms | Improved muscular strength of both lower limbs, chest discomfort, and frequent urination | No mention |
| Heo et al. (19) | Korea Case report | Gagamsamsoeum (加減蔘蘇飮) Hwangnyeonhaedoktang (黃連解毒湯) Yungmijihwangwon ( 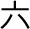 味地黃元) 味地黃元) |
Acupuncture Supportive therapy | No mention | Clinical symptoms | Improved quadriplegia, depression, and insomnia due to frequent urination | No mention |
| Jeon et al. (20) | Korea Case report | Yeongseonjetongeumgagam (靈仙除痛飮加減) Ojeoksan (五積散) Gamiondamtang (加味溫膽湯) |
Acupuncture Bee venom pharmacopuncture Tuina Cupping therapy | No mention | SAEST VAS MMT FSS EDSS | Reduced pain and optic dysfunction -SAEST: F/J: 2>1 → 3>2 H/J: 2 → 3>2 K/J: 2 → 3 - VAS; 9 → 6 - MMT: L/Ex: 2 <3 → 3 - FSS: Sensory func.: 3 → 2 Visual (optic) func.: 4 → 3 - EDSS: 4.0 → 3.0 |
No mention |
| Zhou et al. (21) | China Case-cohort study | Sogangeonbigosubang (疏肝健脾固髓方) |
No mention | Treatment group: acute phase—corticosteroids or gamma globulin; remission phase—herbal medicine Control group: acute phase—corticosteroids or gamma globulin; remission phase—azathioprine |
Recurrence intervals Yearly average recurrence times | Prolonged the recurrence interval - Recurrence interval (p < 0.05) Treatment group: 11.38 ± 8.80 m → 31.76 ± 21.59 m Control group: 12.53 ± 8.23 → 14.26 ± 6.30 Reduced the yearly average recurrence times of MS patients - Yearly average recurrence times (p < 0.05) Treatment group: 2.06 ± 1.94 → 0.56 ± 0.36 Control group: 1.44 ± 0.98 → 1.03 ± 0.49 |
No mention |
| Liu et al. (22) | China Before-and-after study; 1 arm (Bosingosupyeon) | Bosingosupyeon (  ) ) |
No treatment | Among 43 patients, 33 patients: prednisolone acetate 2 patients: carbamazepine |
Clinical symptoms TCM syndrome changes EDSS Evoked potential MRI CSF IgG and oligoclonal zone Serum Myelin basic protein Yearly average recurrence times | Improved symptoms and signs of MS - TER: 88.37% - TCM syndrome changes: 4.37 ± 1.31 → 2.63 ± 1.11 - EDSS: 3.38 ± 1.90 → 2.15 ± 1.55 Reduced recurrent frequency - Yearly average recurrence times (p < 0.05) among 26 patients: 0.99 ± 0.75 → 0.14 ± 0.25 |
None |
| Gao et al. (23) | China Before-and-after study; 2 arms (Jihwang mixture vs patients with functional diseases [such as vascular headache, hysteria, etc.]) | Jihwang mixture (地黄合剂) |
No treatment | Among treatment group, 32 patients; Methylpred- nisolone |
T lymphocyte subset detection (PB and CSF); CD3+, CD4+, CD8+, CD4+/CD8+ EDSS Yearly average recurrence times | Effective against abnormal immune responses in the peripheral blood and central nervous system of MS patients Effectively regulate the immune dysfunction caused by MS - PB/CSF T lymphocyte subset: Treatment group: CD3+: 65.52 ± 5.75/78.15 ± 6.41 → 65.78 ± 5.36/76.56 ± 6.23 CD4+: 44.91 ± 3.93/58.56 ± 5.14 → 46.21 ± 3.85/54.73 ± 4.72 CD8+: 23.50 ± 3.28/28.46 ± 3.53 → 30.26 ± 2.84/30.89 ± 3.17 CD4+/CD8+: 2.27 ± 0.32/2.21 ± 0.35 → 1.76 ± 0.14/1.89 ± 0.21 Control group: CD3+: 66.37 ± 4.30/73.63 ± 3.72 CD4+: 45.63 ± 4.18/51.39 ± 4.18 CD8+: 34.47 ± 1.91/30.89 ± 3.17 CD4+/CD8+: 1.58 ± 0.30/1.89 ± 0.21 - EDSS: 1.02 ± 0.67 → 0.16 ± 0.23 - Yearly average recurrence times: 1.02 ± 0.67 → 0.16 ± 0.23 |
None |
| Etemadifar et al. (24) | Iran RCT: 2 arms (Korean ginseng tablet vs. placebo tablet) | Treatment group: Korean ginseng tablet Control group: Placebo tablet |
No treatment | No treatment | MFIS MSQOL | Reduced fatigue - MFIS: 31.69 ± 14.9 → 23.65 ± 12.8 Improved the quality of life - MSQOL: 53.64 ± 15.51 → 73.56 ± 13.27 |
None |
| Zhou et al. (25) | China RCT: 2 arms (Ihwangbang + methylprednisolone pulse therapy vs. methylprednisolone only) | Treatment group: Ihwangbang (二黃方) Control group: No treatment |
No treatment | Treatment group: acute phase—methylprednisolone; remission phase—herbal medicine Control group: acute phase—methylprednisolone; remission phase—No treatment |
NAA/Cr Relapse rate Annual relapse rate EDSS | Reduced relapse rate and annual relapse rate Prevented progression of MS, which is an effective therapy for relapsing MS - NAA/Cr: Affected side: 1.97 ± 0.59 → 1.77 ± 0.47 Contralateral side: 1.93 ± 0.45 → 1.88 ± 0.50 - Relapse rate: 2.30 ± 1.55 → 0.63 ± 0.58 - Annual relapse rate: 1.01 ± 0.52 → 0.31 ± 0.29 |
No mention |
Cr, creatinine; CSF, cerebrospinal fluid; EDSS, expanded disability status scale; FSS, functional system scale; func., function; F/J, finger joint; H/J, hip joint; Lt., left; K/J, knee joint; L/Ex, lower extremity; m, months; MFIS, modified fatigue impact scale; MMT, manual muscle test; MRI, magnetic resonance imaging; MS, multiple sclerosis; MSQOL, multiple sclerosis quality of life questionnaire; NAA, N-acetylaspartate; PB, peripheral blood; RCT, randomize controlled trial; Rt., right; SAEST, standard for assessment of the effect of stroke treatment; S/J, shoulder joint; TER, total effective rate; U/Ex, upper extremity; VAS, visual analog scale.
