Abstract
Background
Intestinal ultrasound (IUS) is a valid cross-sectional imaging technique for the evaluation of Crohn’s disease (CD). With advancements in technology, portable ultrasound systems are becoming widely available, and the inevitable change to their use by non-radiologist clinicians would be a valuable contribution to improving patient care. This study aimed to investigate the diagnostic yield of IUS examination performed by a gastroenterologist with a portable system as an adjunct imaging modality in the routine care of CD patients.
Methods
A total of 117 CD patients were assessed by IUS imaging. Pre- and post-IUS clinical-management decisions were recorded. The primary outcome was to evaluate the change in the patients’ clinical-management decision following the IUS examination. The diagnostic accuracy was compared against the reference decision reached via a multidisiplinary meeting after the evaluation of all patient-related data. The endoscopic disease activity was determined using the simple endoscopic score for Crohn's disease (SES-CD).
Results
The initial clinical-management decision was changed in 47 patients (40.2%) after the IUS examination (P = 0.001). The accuracy of patient-management decisions improved from 63.2% to 90.6% in comparison to reference decisions (P < 0.001). After IUS examination, a further 13 cases (11.1%) were identified for urgent surgical/interventional procedures. The accuracy of colonoscopic (SES-CD ≥3) assessment was shown to be comparable to that of IUS (94% vs 91%). The sensitivity for disease presence was 95% with colonoscopy and 94% with the IUS assessment.
Conclusion
IUS examination with the use of a portable ultrasonography system significantly improves clinical-management decisions. With further supporting data, this practice would possibly become a requirement for CD management.
Keywords: Crohn’s disease, inflammatory bowel diseases, point-of-care, portable, ultrasound, monitoring
Introduction
Crohn’s disease (CD) is a chronic, relapsing form of inflammatory bowel disease (IBD) that could affect any portion of the gastrointestinal tract and is characterized by transmural inflammation that is interspersed with healthy sections of bowel. The disease is progressive; uncontrolled inflammation could result in stricturing or penetrating complications. Imaging techniques play a fundamental role in the initial diagnostic work-up (to determine the extent and location of CD), subsequent monitoring of disease activity, and the detection of complications in CD [1].
Conventional fluoroscopy-based imaging techniques including small-bowel follow-through and/or enteroclysis have largely been replaced by cross-sectional imaging techniques including computed tomography (CT), magnetic resonance (MR) imaging, and ultrasound. Cross-sectional imaging modalities have proved to be of value in detecting extraluminal complications in addition to mural involvement. Repeated imaging studies over time are needed for most CD patients and concerns about radiation exposure have limited the use of CT enterography. The selection of the appropriate imaging modality depends on its local availability, patient preferences, cost, and reimbursement issues. Expertise required for performing these assessments and technical details of these imaging techniques is also subject to considerable variation, which may affect accuracy.
Intestinal ultrasound (IUS) has become an increasingly used diagnostic modality for the management of CD patients due to its high sensitivity and specificity in the detection of intestinal inflammatory activity and disease extent [2]. In a recent report, high sensitivity for detecting endoscopic activity was further demonstrated in a heterogeneous patient cohort with different disease phenotypes, and its potential to reduce the number of routine ileocolonoscopies for patient monitoring was suggested [3].
IUS has several advantages when compared to other cross-sectional imaging modalities. It is inexpensive, non-invasive, well tolerated, and broadly available, and confers no radiation exposure [4]. The development of portable ultrasound systems has enabled clinicians to perform ultrasound, which provides a snapshot of the disease status when required. Furthermore, it permits repeated examination during the disease course to guide the patient’s management [5]. When performed by an IBD specialist, it may be advantageous for patient care, as IUS gives the opportunity to interpret the findings in real time within the particular clinical scenario and limits unnecessary radiology-department visits. However, data regarding the influence of routine IUS for clinical decision-making is sparse and no study evaluating the diagnostic capability of portable ultrasound systems has been published. The aim of this study was to investigate the diagnostic yield of routine IUS performed by the gastroenterologist with a portable ultrasound machine in the management of CD patients.
Patients and methods
Data sources
Adult CD patients (age >18 years, with a known diagnosis for at least 6 months, inpatient or outpatient) who consecutively attended Haydarpasa Numune Training and Research Hospital gastroenterology and general surgery clinics between 2015 and 2018 were prospectively evaluated by utilizing a portable ultrasound system in addition to their routine follow-up. Disease duration, localization, and behavior according to Montreal classification [6]; IUS findings; drugs used for treatment; and disease-related surgical history were recorded for all CD patients in both clinics. Colonoscopic examinations were performed within 4 weeks following the IUS. The Harvey-Bradshaw Index (HBI) [7] and simple endoscopic score for Crohn’s disease (SES-CD) [8] were used for clinical- and endoscopic-activity assessments, respectively. HBI scores ≥5 and SES-CD scores ≥3 were set as cut-offs for clinical and endoscopic activity, respectively. The results of laboratory tests, cross-sectional imaging reports, and all relevant data were retrieved from the records stored in the Haydarpasa Numune Training and Research Hospital database. Laboratory test results (C-reactive protein) obtained within a 1-week period of the IUS examinations were used for outcome analysis. The exclusion criteria included age <18 years, pregnancy, isolated upper-gastrointestinal involvement, and severe co-morbidities or intercurrent diseases precluding colonoscopic examination. The study was conducted in accordance with the Helsinki Declaration and approved by the Haydarpasa Numune Training and Research Hospital Clinical Research Ethics Committee (HNEAH-KAEK 2020/71).
Technique of IUS
All ultrasound examinations were performed by the same gastroenterologist using the LOGICTMe Ultrasound System (GE Healthcare, USA) using convex C1-5-RS (2.0–5.0 MHz) and linear array (9 L-RS [3.3–10 MHz] or 12 L-RS [4.2–13 MHz]) transducers. The LOGICTMe Ultrasound System is laptop-style, compact, portable ultrasound system optimized for point-of-care applications. A standardized protocol was used for all ultrasonographic examinations. Briefly, examination started in the right iliac fossa by identifying the terminal ileum and caecum, and proceeded from the caecum to the rectum. If not done previously, standard abdominal ultrasound examination was added to the procedure for the detection of any additional intra-abdominal pathology. IUS examinations were performed without any preparations such as fasting or oral intake of fluids. Perineal ultrasonography was undertaken at the clinician’s discretion for the patients with known perianal involvement.
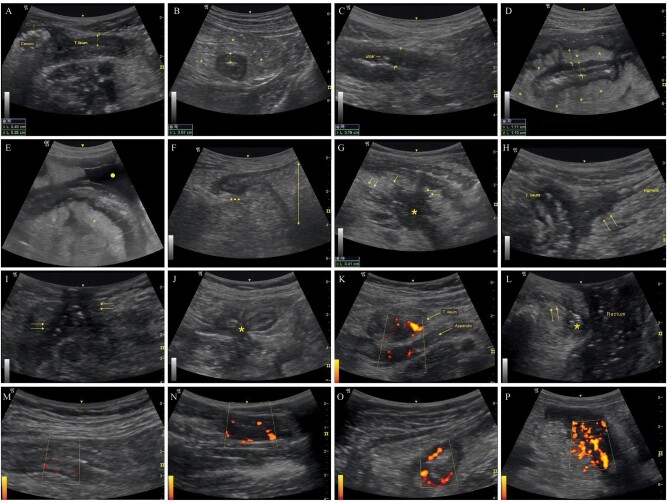
During the ultrasound examination, the following parameters were noted for each patient: bowel-wall thickness (BWT), mesenteric fibrofatty proliferation (>5 mm), stratification (no stratification-transmural involvement, stratified pattern, or mixed), stricture, prestenotic dilatation (>3 cm), rigidity, lymphadenopathy, fistula, phlegmon/abscess, and fluid collections (Figure 1). BWT ≥3 mm was set as a cut-off for thickening. Vascularity within the affected bowel wall was semiquantitatively determined by power Doppler using a modified Limberg score [9]. Obvious vascularity with scores ≥2 was considered a sign of active disease (Figure 1).
Figure 1.
Representative ultrasonographic findings. (A) Thickened terminal ileal wall (thickness: 4.9 mm) compared to cecal wall. (B) Thickened (5.7 mm) intestinal wall in transverse section and reactive hyperechoic mesentery (*). (C) Ulcer located in the affected wall showing transmural involvement. (D) Thickened (11 mm) terminal ileal wall with stratified involvement and mesenteric fibrofatty proliferation (*). (E) Reactive mesentery (*) around diseased terminal ileal segment with accompanying fluid (circle). (F) Prestenotic dilatation (double-headed arrow) proximal to the diseased segment (arrowheads). (G) Abscess (*) and related fistulae (arrows) originating from the diseased ileal segment with stratified involvement. (H) Fistulous tract between the terminal ileum and sigmoid colon (arrows). (I) Enterocutaneous fistulous tracts (arrows) with hyperechoic air inside. (J) Enteroenteric fistulae involving adjacent intestinal loops with center shrinkage (star sign). (K) Appendiceal involvement showing thickened terminal ileum and appendix with accompanying vascularity. (L) Transperineal ultrasound showing abscess (*) related to the fistulae (arrows) extending from the anal canal to the skin. (M) Limberg grade 1: Barely visible vascularity in the intestinal wall. (N) Limberg grade 2: Obvious vascularity as prominent vascular spots. (O) Limberg grade 3: Longer stretches of vascularity in the involved segment. (P) Limberg grade 4: Vascularity reaching mesentery.
Outcome measurements
The clinical-management decision by the referring physician was recorded before conducting the IUS procedure (the Pre-IUS group). Thereafter, IUS was performed by the gastroenterologist who was blinded to the pre-IUS decision within 3 days and made an independent decision (the Post-IUS group). No additional imaging or laboratory investigations were done between the two decisions. All physicians (including the IUS operator) were not blind to any data (including clinical data, physical examination, laboratory values, previous endoscopic/imaging results) regarding the patient. The decision could lead to any of the following treatments: (i) no change in the treatment plan, (ii) treatment de-escalation, (iii) treatment escalation (including dose increase, additional drug initiation, or drug switch), (iv) further imaging/endoscopy (such as MR enterography, colonoscopy), (v) referral for a therapeutic procedure or surgery (including endoscopic dilatation of a stricture, large- and small-bowel surgery as well as perianal surgery), and (vi) combination (medical-treatment escalation plus (iv) and/or (v)). After the completion of all diagnostic and therapeutic interventions, each patient was discussed in a multidisciplinary meeting (MDM) that was held no later than 3 months after the initial IUS. During the MDM, results of all radiologic examinations including IUS, CT, and MR enterography (MRE); endoscopic evaluations; surgical findings; pathology results; laboratory tests (C-reactive protein, fecal calprotectin levels, therapeutic drug measurement results) along with clinical data (activity indexes, course) were discussed. During the MDM, a mutual construct reference decision was reached, which is regarded as the gold standard. MDM decisions regarding patient management were taken in accordance with the European Crohn’s and Colitis Organisation (ECCO) guidelines. Pre-IUS and post-IUS decisions were retrospectively compared against the decision reached during the MDM. The accuracy of the IUS-based decisions was determined.
Statistical analysis
Standard descriptive statistics were used to characterize the sample and the McNemar test was used for comparing paired proportions. The marginal homogeneity test was preferred when more than two variable numbers were present. The Cochran’s Q test was selected to determine whether there were differences on a dichotomous dependent variable between three or more related groups. Strength and direction of association between two ranked variables were analysed by using Spearman’s rank-order correlation. P < 0.05 was considered statistically significant. The Statistical Package for Social Sciences (SPSS, SPSS Inc., Chicago, IL, USA), version 22.0 for Windows, was used to analyse the data. The accuracy of pre- and post-IUS clinical decisions were calculated as the percentage of correctly classified cases using the formula “(true positive + true negative)/total number of population”.
After sample-size calculations, it was ascertained that an enrollment of 85 patients into the study was required for a statistical power of 80% to detect an increase of 15% in the rate of clinical-decision change after IUS, with P < 0.05. In a previous study, it was demonstrated that the IUS practice increased “treatment change/imaging need” from 37% and 49% (for two researchers, respectively) to 69% [10]. Due to the considerable variation in patient-population characteristics, practice and remission targets, “treatment change/imaging need” ranged from 20% to 50%, and ≤80% in other studies with corresponding sample sizes of 62, 85, and 42 for these wide baseline ratios, respectively [10, 11].
Results
Demographics and clinical characteristics
One hundred and seventeen patients were included in the study. The mean age was 37.7 ± 14.1 years, ranging from 18 to 75. Of 117 patients, 66 patients (56.4%) were male. The distribution of inflammatory, stricturing, and penetrating behavior according to Montreal classification were 42.7%, 22.2%, and 35.0%, respectively. Patients under biologic, immunomodulatory, or combination drug therapy represented 68.4% of the study population. Patient numbers with HBI scores ≥5 and SES-CD scores ≥3 were 78 (66.7%) and 97 (82.9%), respectively. The characteristics of the study patients are depicted in Table 1.
Table 1.
Demographic and baseline characteristics of 117 patients with Crohn’s disease
| Characteristic | Value |
|---|---|
| Age, years | 37.7 ± 14.1 |
| Female gender | 51 (43.6) |
| Body mass index (kg/m2) | 22.3 ± 3.4 |
| Smoking (current/former) | 43 (36.8)/15 (12.8) |
| Alcohol user | 11 (9.4%) |
| Disease duration, months (25th–75th percentiles) | 36 (6–76) |
| Age at diagnosis | |
| A1 (<17 years) | 10 (8.5) |
| A2 (17–40 years) | 77 (65.8) |
| A3 (>40 years) | 30 (25.6) |
| Disease location | |
| L1 (Ileal) | 29 (24.8) |
| L2 (Colonic) | 22 (18.8) |
| L3 (Ileocolonic) | 66 (56.4) |
| Upper-gastrointestinal involvement | 25 (21.4) |
| Disease behavior | |
| B1 (Non-stricturing, non-penetrating) | 50 (42.7) |
| B2 (Stricturing) | 26 (22.2) |
| B3 (Penetrating) | 41 (35.0) |
| p (perianal involvement) | 23 (19.7) |
| Surgical history | 31 (26.5) |
| Ileocecal resection | 15 (12.8) |
| Seton placement | 8 (6.8) |
| Small-bowel or colonic resection/stricturoplasty | 6 (5.1) |
| Multiple | 3 (2.6) |
| Medications | |
| Mesalazine | 37 (31.6) |
| Monotherapy with IMM (Azathioprine/Methotrexate) | 40 (34.2) |
| Biologics (Mono) | 11 (9.4) |
| Combo treatment (Biologic + IMM) | 29 (24.8) |
| Steroids (Conventional/budesonide, past or current user) | 86 (73.5) |
| Harvey-Bradshaw Index (25th–75th percentiles) | 6 (2.5–9.0) |
| No. of patients with score <5 | 39 (33.3) |
| No. of patients with score ≥5 | 78 (66.7) |
| C-reactive protein, nmol/L (25th–75th percentiles) | 13.8 (3.4–49.5) |
| No. of patients with CRP ≥4.8 | 76 (65.0) |
| Total SES-CD score (25th–75th percentiles) | 7 (3.0–11.5) |
| Absence of mucosal remission (SES-CD ≥3) | 97 (82.9) |
Data are presented as mean ± standard deviation, median (25th–75th percentiles), or n (%).
CRP, C-reactive protein; IMM, immunomodulator; SES-CD, simple endoscopic score for Crohn’s disease.
Interventions and outcomes
In 47 patients (40.2%), management decisions were changed after IUS examination (P = 0.001, marginal homogeneity test). Moreover, “treatment escalation,” “therapeutic/surgical intervention,” and “combined” approach rates increased following the IUS examination, with a reciprocal decrease in “no change in treatment” and “further imaging” decisions (Figure 2). There was a significant difference in the accuracy of clinical decisions before (74/117, 63.2%) and after IUS (106/117, 90.6%), when the gold standard was applied as a reference (P < 0.001, McNemar Test).
Figure 2.
Pre- and post-intestinal-ultrasound decisions. (A) Number of patients in each decision group. (B) Directions of changes between groups. Numbers of patients leaving and entering individual decision groups are shown in circles.
After IUS, 13 (11.1%) additional cases were referred for surgical/interventional procedures due to abscess/phlegmon (n = 7), structuring disease with enteroenteric fistulas (n = 2), fistula related to the urinary system (impending ileovesical fistula and recto-urethra-scrotal fistula; n = 2), and structuring disease with prestenotic dilatation (n = 2). For all cases, symptoms were considered to be associated with inflammatory-type disease before IUS. Thus, the pre-IUS clinical-management decisions for patients were treatment escalation or further imaging. The utilization of IUS in clinical practice prevented treatment escalation by identifying abscesses or disease complications that otherwise could have had detrimental results. Although further imaging could also be an alternative option for identifying some of these complications, IUS examination played a critical role in accelerating the triage of these cases.
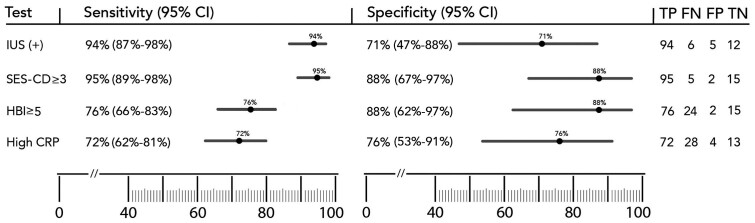
The sensitivity and specificity of (any) abnormal IUS-examination findings for clinical-management decision-making were 94% (95% confidential interval [CI], 87%–98%) and 70.6% (95% CI, 47%–88%), respectively. Sensitivity and specificity of SES-CD ≥3 were calculated as 95% (95% CI, 89%–98%) and 88% (95% CI, 67%–97%), which were comparable to those of IUS (Figure 3). In five patients, diseased segments were out of reach for the colonoscope. Mucosal lesions identified during colonoscopy were not related to disease recurrence in two patients (rather infection was identified in one and a non-steroidal anti-inflammatory drug used in the other). Sensitivity and specificity values for clinical-activity scoring (HBI ≥5) were 76% (95% CI, 66%–83%) and 88% (95% CI, 62%–97%), respectively. Corresponding values for elevated C-reactive protein (CRP) levels were 72% (95% CI, 62%–81%) and 76% (95% CI, 53%–91%). The accuracy of colonoscopic-activity (SES-CD ≥3) assessments, IUS positivity, clinical activity (HBI ≥5), and high CRP levels (≥4.8 nmol/L) were 94%, 91%, 77%, and 73%, respectively (P < 0.001, Cochran’s Q test). The accuracy of colonoscopic-activity assessment was comparable to that for IUS positivity. However, it was statistically superior when compared with clinical activity (P < 0.001, McNemar Test) and CRP elevation (P < 0.001, McNemar Test).
Figure 3.
Sensitivity and specificity of intestinal-ultrasound positivity, colonoscopic activity, clinical activity, and high C-reactive protein against the reference standard
Error bars represent 95% CI.
CI, confidential interval; CRP, C-reactive protein; FN, false negative; FP, false positive; HBI, Harvey-Bradshaw Index; IUS, intestinal ultrasound; TN, true negative; TP, true positive; SES-CD, simple endoscopic score for Crohn’s disease.
Increased BWT was the most common IUS finding (Table 2). Other parameters were not observed without increased BWT, except in one case in which a rigid ulcerated stricture with prestenotic dilatation and surrounding mesenteric fibrofatty proliferation was identified. Correlation between BWT and the SES-CD was found to be moderate to good except in the rectum, where measurements in general were not reliable because of the distance between the ultrasound transducer and the rectum. Corresponding Spearman’s rank correlation values are depicted in Table 3. In five patients (including two post-operative cases), the IUS examination revealed thickened bowel-wall segments without mucosal disease. In three patients without a history of any surgical operation, the increase in BWT might be the result of unresolved permanent transmural structural damage despite effective treatment. The second most common IUS finding was mesenteric fibrofatty proliferation (73.5%). Other findings are listed in Table 2. The IUS examination revealed two cases (1.7%) with appendicular involvement (Figure 1). Although upper-gastrointestinal involvement was not systematically investigated by ultrasound, one case with gastric sub-epithelial mass-like involvement was identified [12].
Table 2.
Comparison of intestinal-ultrasound findings between patients with or without decision changes
| Ultrasonographic finding | All patients | Patients with decision change after IUS (n = 47) | Patients without decision change after IUS (n = 70) | P-value |
|---|---|---|---|---|
| Bowel-wall segments with thickening ≥3 mm | ||||
| Terminal ileum | 83 (70.9) | 36 (76.6) | 47 (67.1) | <0.001 |
| Right colon | 54 (46.2) | 20 (42.6) | 34 (48.6) | ns |
| Transverse colon | 33 (28.2) | 7 (14.9) | 26 (37.1) | ns |
| Left-colon sigmoid | 35 (29.9) | 10 (21.3) | 25 (35.7) | ns |
| Rectum | 7 (6) | 0 | 7 (10) | <0.001 |
| Any bowel segment with thickening ≥3 mm | 98 (83.8) | 40 (85.1) | 58 (82.9) | <0.001 |
| Involvement pattern | <0.001 | |||
| Transmural | 35 (29.9) | 19 (40.4) | 16 (22.9) | |
| Mixed (stratified and transmural) | 38 (32.5) | 14 (29.8) | 24 (34.3) | |
| MFP | 86 (73.5) | 37 (78.7) | 49 (70) | <0.001 |
| Ulcer presence | 66 (56.4) | 29 (61.7) | 37 (52.9) | 0.014 |
| Strictures | 32 (27.4) | 22 (46.8) | 10 (14.3) | 0.017 |
| Prestenotic dilatation | 20 (17.1) | 13 (27.7) | 7 (10) | <0.001 |
| Rigidity | 37 (31.6) | 23 (48.9) | 14 (20) | ns |
| Lymphadenopathy | 38 (32.5) | 18 (38.3) | 20 (28.6) | ns |
| Fistula | 22 (18.8) | 11 (23.4) | 11 (15.7) | <0.001 |
| Phlegmon/abscess | 16 (13.7) | 9 (19.1) | 7 (10) | <0.001 |
| Fluid | 19 (16.2) | 12 (25.5) | 7 (10) | <0.001 |
| Power Doppler score ≥2 | 71 (60.7) | 30 (63.8) | 41 (58.6) | 0.002 |
| Any sign of disease activity on ultrasound | 99 (84.6) | 41 (87.2) | 58 (82.9) | <0.001 |
Data are presented as n (%).
For comparisons between patients with or without decision changes, the McNemar test was used except for “involvement-pattern” comparisons, for which the marginal homogeneity test was preferred.
MFP, mesenteric fibrofatty proliferation; ns, non-significant.
Table 3.
Correlation between increased ultrasonographic bowel-wall thickness and colonoscopic activity in different segments of the colon.
| Segment of the colon | Patients with BWT ≥3 mm | Patients with SES-CD ≥3 | r s-value | P-value |
|---|---|---|---|---|
| Ileum | 83 (70.9) | 60 (51.3) | 0.483 | <0.001 |
| Right colon | 54 (46.2) | 53 (45.3) | 0.576 | <0.001 |
| Transverse | 33 (28.2) | 31 (26.5) | 0.660 | <0.001 |
| Left colon | 35 (29.9) | 33 (28.2) | 0.609 | <0.001 |
| Rectum | 7 (6.0) | 12 (10.3) | 0.332 | <0.001 |
Data are presented as n (%).
BWT, bowel-wall thickness; rs, Spearman’s rank correlation coefficient; SES-CD, simple endoscopic score for Crohn's disease.
In 23 post-operative patients (with the exclusion of 8 patients with seton placements), pre-IUS decisions differed in 10 patients when compared to the gold standard. After IUS examination, only three decisions were different in comparison to MDM decisions (P = 0.008, marginal homogeneity test). In six patients with colonoscopic remission, IUS correctly classified four patients as normal. In one patient with ileocecal resection, IUS identified wall thickening at the anastomosis site despite there being no recurrence endoscopically. This could be the result of chronic changes after surgical resection or improper identification of the anastomosis site using IUS. The other false positivity was encountered in a patient with multiple resections that render IUS examination difficult. IUS correctly identified all post-operative recurrences except one case. This false negativity was observed in one patient with ileocecal resection with more than five aphthous ulcerations at the anastomosis site. IUS significantly increased the accuracy of clinical decision-making from 56.5% (13/23) to 87.0% (20/23) in the post-operative patient group, when the gold standard was applied as a reference (P = 0.016, McNemar Test).
Discussion
In the present study, IUS examination performed by a gastroenterologist with a portable system led to a change in the clinical management in 40.2% of cases. Furthermore, there was a substantial improvement from 63.2% to 90.6% in the accuracy of patient-management decisions. The study population closely represented patients in a real-world setting, since it included difficult-to-treat patients with 57.3% having structuring or penetrating behavior, and with >80% having active mucosal disease. The IUS examination also enabled timely triage of patients for the appropriate surgical/interventional procedures.
Ultrasound is an established powerful technique for the diagnosis of suspected CD, the assessment of disease extent, the identification of complications, in addition to the detection of post-operative recurrence [1, 2]. In this study, the IUS examination was conducted using a portable ultrasound system as an adjunct imaging modality for routine follow-up evaluation of CD patients. The diagnostic capability of IUS examination was high, with a sensitivity of 94% and a specificity of 70.6%, which are in accordance with literature evaluating the performance of the ultrasonographic technique using conventional ultrasound systems in different clinical scenarios [2]. In a recent study using conventional high-tech ultrasound, Sævik et al. [3] demonstrated that IUS achieved a sensitivity of 92.2% and a specificity of 86% in evaluating disease activity, in which mucosal healing was strictly defined as SES-CD = 0. Based on this high sensitivity, authors suggested that patients with active disease on IUS do not need an ileocolonoscopy as the false-positivity rate was low for IUS; however, patients with ileocolonic disease and normal findings on IUS should be examined using ileocolonoscopy to exclude false negativities. Our results also support this concept, enabling “rational use” of endoscopic resources.
In a previous study including 49 patients, the IUS examination was performed using a conventional high-resolution ultrasound system leading to changes in the clinical-management decisions in 60% of the cases evaluated [10]. The asymptomatic patient rate was reported as 59%, but the accuracy of IUS examination was not compared to that of colonoscopy or any other gold standard. The rate of change in clinical-management decisions reported in that study was higher than that in our study. However, the impact of intestinal ultrasonography for patient management was associated with substantial changes in medication usage and the surgical-consultation rate, and also a decrease in further-imaging demands, revealing similar trends in both studies (Figure 2).
With the advancement of portable ultrasound machines, healthcare professionals from nearly all clinical specialties have begun to utilize point-of-care ultrasonography in various clinical scenarios, with a focused clinical question and goal in mind. In the present study, the use of portable laptop-style ultrasound decreased imaging needs (Figure 2). This “sparing effect” of portable IUS practice for conventional machine-based ultrasound imaging renders patient transfers to remotely located radiology clinics unnecessary. Further technological improvements resulting in high-resolution imaging, pocket-sized or mobile-phone-compatible transducers, and advancements in artificial intelligence would probably render ultrasonography as the “visual stethoscope” of practicing gastroenterologists in the near future [5].
Progression of bowel damage is an intrinsic characteristic of CD, and this natural evolution can occur despite the use of optimal medical therapy. Therefore, timely decision-making in relation to surgery is undoubtedly of great importance for complicated cases. In the present study, 13 (11.1%) additional cases were referred for surgical/interventional procedures after IUS examination (Figure 2). In this group, IUS practice prevented the loss of valuable time related to ineffective treatments that otherwise could have had resulted in a more complex disease phenotype, limiting the action of minimally invasive surgery due to extensive disease, infectious complications, involvement of adjacent healthy small-bowel segments, and fistulas.
Relying upon patient symptoms to guide therapy can lead to critical errors in the management of CD owing to the discrepancy between endoscopic activity and clinical indices. In 18%–20% of cases, symptoms are not related to any significant mucosal disease, and nearly half of patients in clinical remission have endoscopic activity according to the literature [13, 14]. Furthermore, the diagnostic accuracy of CRP as a biomarker of inflammation for patient monitoring is highly diverse, and far from perfect [15]. In the present study, 24 patients with HBI <5 and 28 patients with normal CRP levels were found to have active disease. In contrast, only six patients with active disease were not identified by the IUS assessment. The accuracy of colonoscopic-activity assessment (94%) was found to be comparable to that of IUS examination (91%). Furthermore, the IUS assessment affected patient management in five additional cases in which the disease was located out of reach of the colonoscope, thereby demonstrating its important role as a complementary modality to the “gold-standard” ileocolonoscopy.
In recent years, the emphasis of CD treatment has had a paradigm switch from a conventional step-up approach to early aggressive control of inflammation to reduce bowel damage, targeting mucosal healing as a therapeutic endpoint [16]. This “treat-to-target” concept involves multiple assessments including endoscopy, biomarkers analysis, and imaging modalities. Furthermore, transmural healing is becoming a reasonable therapeutic target and still under investigation to define its role in the concept of deep remission [17]. MRE is the preferred imaging modality in general because of its higher sensitivity and specificity in comparison to ultrasonography. In a recently published METRIC trial, MRE showed 97% sensitivity and 96% specificity with respect to disease presence. The corresponding values for ultrasonography were 92% and 84% [18]. However, repeated MR imaging results in high health expenditures alongside delays caused by its lengthy appointment waiting times that are usually associated with treatment delays. The IUS assessment offers a fast, reliable, and inexpensive alternative to MRE modality. The IUS can be considered as the initial point-of-care modality, since patient preference is low and its preparation is bothersome for ileocolonoscopy. Low patient acceptability is also a limitation for MRE because of the longer recovery time in contrast to that of IUS [19].
In the present study, there was a moderate to good correlation between BWT and SES-CD, except that of the rectum (Table 3). Studies using high-resolution conventional ultrasound systems reported slightly superior correlation between BWT and SES-CD, which also depends on the ultrasonographic BWT cut-off (3 or 4 mm) used or the definition of colonoscopic activity (SES-CD 0 or <3) [3]. Increased BWT seems the most important difference between patients with active disease and those in remission in our study, which is compatible with previous reports [2–4]. Other IUS findings can further change management decisions by identifying a more complicated course (such as fistula, abscess, or stenosis). Doppler measurements performed on pathologically thickened bowel walls should be considered for disease-activity quantification, supporting previous reports [3, 4]. Furthermore, a recently published TRUST study showed improvement of IUS parameters after treatment, promising the usefulness of IUS as an alternative and fast imaging modality to determine transmural healing [20]. The role of portable IUS practice as an essential component of the “treat-to-target” approach and combined use with other biomarkers to reach strict CD control should be determined using additional studies.
IUS has also proved to be a valid method for detecting post-operative recurrence and correlates well with ileocolonoscopy [2, 4]. However, evaluating the anastomosis site would be difficult due to chronic changes related to surgery and could have been the cause of two false positives in our study. IUS substantially increased the accuracy of decision-making from 56% to 87%. Although our study population includes a small number of post-operative patients with heterogeneous surgical history, this high accuracy supports its role as an adjunct method for routine patient care in post-operative CD patients.
In some European countries, theoretical and practical training in abdominal ultrasonography is an integral part of gastroenterology-training programs, with gastroenterologists commonly performing IUS assessments. In contrast, in other parts of the world like North America, ultrasonography is performed by radiology clinics. Guidelines regarding the application of imaging modalities in CD also reflect the aforementioned discrepancy [21–23]. ECCO/ESGAR guidelines recommend IUS or MRE for the monitoring of clinically symptomatic patients and for the detection of complications [1]. In Asian and North American guidelines, IUS was not prioritized when compared to MRE or CTE [22, 23]. Indian guidelines particularly emphasizes the use of contrast-enhanced ultrasonography for the differentiation of inflammatory and fibrotic strictures [24].
Our study has several limitations. First, MRE examinations were not routinely performed, as transmural healing was not the priority of the patient’s clinical management when the study was designed. Therefore, performing MRE imaging was not considered necessary for inactive patients during most of the study time period. Given the substantial number of studies comparing MRE imaging with IUS imaging, our study did not aim to compare different radiologic modalities in a strictly controlled population, but to determine the yield of ultrasonographic examination performed using a portable system in a real-life setting. The portable ultrasonography system used in this study lacked elastography and contrast-enhanced ultrasonography modalities, both of which can increase the diagnostic capability of ultrasonographic imaging [25, 26]. The addition of these modalities into portable systems may enhance the system’s accuracy in the future. Second, this study was not designed to evaluate changes in IUS findings post treatment. It has been estimated that a larger study population would be required for this purpose on the basis of different treatment options and the high number of drug options [20]. Third, all ultrasound examinations were performed by the same gastroenterologist in the present study and no intra- or inter-observer analysis was performed. However, it is reported that the inter-observer agreement of IUS is good to excellent in both newly diagnosed and relapsing patients [3, 27].
In conclusion, this study offers real-life evidence of the efficacy of IUS assessment conducted using a portable system for clinical decision-making in CD patients. The ability to visualize the disease status in real time using point-of-care ultrasound can provide rapid, high-quality, robust, and cost-effective patient care. As new technical advancements emerge, our understanding of how to integrate portable ultrasonography imaging modality into IBD clinical practice will expand, and the routine use of this imaging technique will be incorporated as a new standard of care.
Authors’ contributions
C.G. and A.S. designed the study. C.G., A.S., K.K., S.O., E.A., and M.T. collected and analysed the data. C.G. performed the procedures and statistical analysis. C.G., A.S., K.K., S.O., E.A., and M.T. drafted and critically revised all the intellectual content of the manuscript. All authors read and approved the final manuscript.
Funding
No specific funding has been received for this study.
Conflicts of interest
None declared.
References
- 1. Maaser C, Sturm A, Vavricka SR. et al. ; European Crohn’s and Colitis Organisation (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR). ECCO-ESGAR guideline for diagnostic assessment in IBD. Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–64. [DOI] [PubMed] [Google Scholar]
- 2. Calabrese E, Maaser C, Zorzi F. et al. Bowel ultrasonography in the management of Crohn's disease: a review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016;22:1168–83. [DOI] [PubMed] [Google Scholar]
- 3. Sævik F, Gilja OH, Nylund K.. Gastrointestinal ultrasound can predict endoscopic activity in Crohn's disease. Ultraschall Med 2020, 10.1055/a-1149-9092. [DOI] [PubMed] [Google Scholar]
- 4. Kucharzik T, Kannengiesser K, Petersen F.. The use of ultrasound in inflammatory bowel disease. Ann Gastroenterol 2017;30:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhagra A, Tierney DM, Sekiguchi H. et al. Point-of-care ultrasonography for primary care physicians and general internists. Mayo Clin Proc 2016;91:1811–27. [DOI] [PubMed] [Google Scholar]
- 6. Silverberg MS, Satsangi J, Ahmad T. et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 7. Vermeire S, Schreiber S, Sandborn WJ. et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol 2010;8:357–63. [DOI] [PubMed] [Google Scholar]
- 8. Daperno M, D'Haens G, Van Assche G. et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 9. Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol 1999;37:495–508. [PubMed] [Google Scholar]
- 10. Novak K, Tanyingoh D, Petersen F. et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn's disease: impact on clinical decision making. ECCOJC 2015;9:795–801. [DOI] [PubMed] [Google Scholar]
- 11. Colombel JF, Panaccione R, Bossuyt P. et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 12. Gonen C, Ozkara S.. Gastric subepithelial mass-like involvement in Crohn's disease: another upper gastrointestinal manifestation. J Crohns Colitis 2018;12:262–3. [DOI] [PubMed] [Google Scholar]
- 13. Peyrin-Biroulet L, Reinisch W, Colombel JF. et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 2014;63:88–95. [DOI] [PubMed] [Google Scholar]
- 14. Rutgeerts P, Diamond RH, Bala M. et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc 2006;63:433–42. [DOI] [PubMed] [Google Scholar]
- 15. Mosli MH, Zou G, Garg SK. et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19. [DOI] [PubMed] [Google Scholar]
- 16. Khanna R, Bressler B, Levesque BG. et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet 2015;386:1825–34. [DOI] [PubMed] [Google Scholar]
- 17. Panés J, Rimola J.. Is the objective of treatment for Crohn's disease mucosal or transmural healing? Clin Gastroenterol Hepatol 2018;16:1037–9. [DOI] [PubMed] [Google Scholar]
- 18. Miles A, Bhatnagar G, Halligan S. et al. ; on behalf of the METRIC investigators. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn's disease: patient acceptability and perceived burden. Eur Radiol 2019;29:1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor SA, Mallett S, Bhatnagar G. et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol 2018;3:548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kucharzik T, Wittig BM, Helwig U. et al. Use of intestinal ultrasound to monitor Crohn's disease activity. Clin Gastroenterol Hepatol 2017;15:535–42. [DOI] [PubMed] [Google Scholar]
- 21. Taylor SA, Rieder F, Fletcher JG.. Differences in the imaging of Crohn's disease patients between North America and Europe: are we ready to bridge the divide? Abdom Radiol 2019;44:1637–43. [DOI] [PubMed] [Google Scholar]
- 22. Lichtenstein GR, Loftus EV, Isaacs KL. et al. ACG clinical guideline: management of Crohn's disease in adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 23. Ooi CJ, Makharia GK, Hilmi I. et al. ; Asia Pacific Association of Gastroenterology (APAGE) Working Group on Inflammatory Bowel Disease. Asia Pacific Consensus Statements on Crohn's disease. Part 1: Definition, diagnosis, and epidemiology (Asia Pacific Crohn's Disease Consensus--Part 1). J Gastroenterol Hepatol 2016;31:45–55. [DOI] [PubMed] [Google Scholar]
- 24. Kedia S, Sharma R, Makharia GK. et al. ; for Indian Society of Gastroenterology Task Force on Inflammatory Bowel Disease. Imaging of the small intestine in Crohn's disease: joint position statement of the Indian Society of Gastroenterology and Indian Radiological and Imaging Association. Indian J Gastroenterol 2017;36:487–508. [DOI] [PubMed] [Google Scholar]
- 25. Vestito A, Marasco G, Maconi G. et al. Role of ultrasound elastography in the detection of fibrotic bowel strictures in patients with Crohn's disease: systematic review and meta-analysis. Ultraschall Med 2019;40:646–54. [DOI] [PubMed] [Google Scholar]
- 26. Medellin A, Merrill C, Wilson SR.. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol 2018;43:918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhatnagar G, Quinn L, Higginson A. et al. ; METRIC study investigators. Observer agreement for small bowel ultrasound in Crohn's disease: results from the METRIC trial. Abdom Radiol 2020;45:3036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]