FIGURE 2.
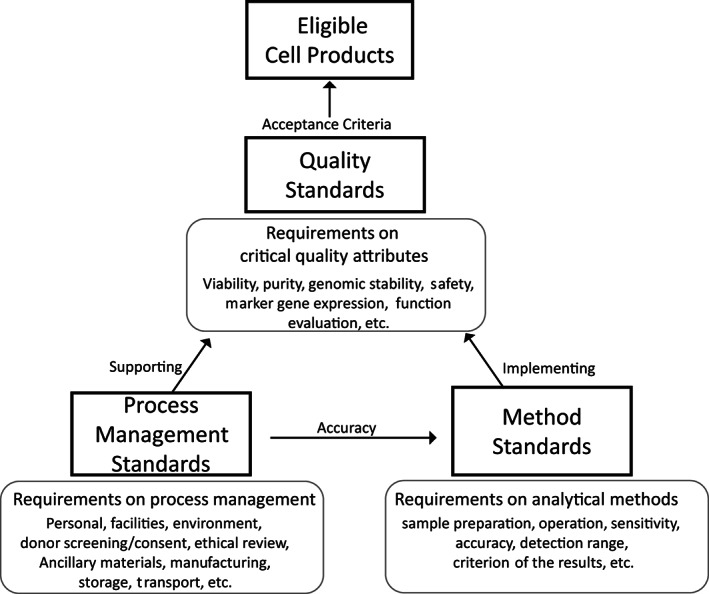
Stem cell standard systems. Quality standards specify the acceptance criteria of critical quality attributes of stem cell products, separating the acceptability from unacceptability. Method standards, which specify the requirements for evaluation of the critical quality attributes of cell products, facilitate implementation of quality standards. Process standards specify the management requirements supporting the whole operating process, thus ensuring the accuracy of testing results and quality of final cell products
