FIGURE 1.
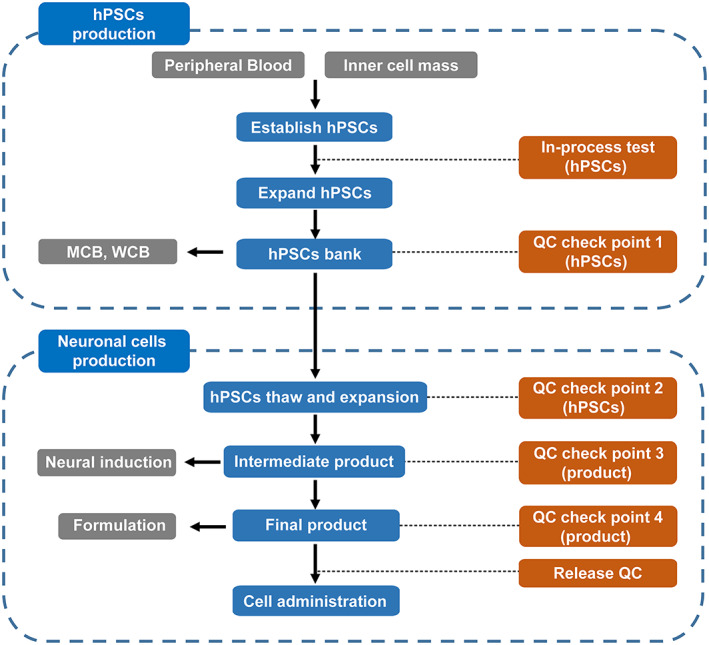
An overview of manufacturing workflow of clinical neuronal cells. In‐process test (hPSCs): morphology, karyotype, sterility, mycoplasma, viral. QC check point 1 (hPSCs): morphology, karyotype, sterility, mycoplasma, viral, cell number, viability, endotoxin, hPSCs marker expression, three germ layers formation ability. QC check point 2 (hPSCs): morphology, karyotype, sterility, mycoplasma, cell number, viability, endotoxin, hPSCs marker expression. QC check point 3 (intermediate product): morphology, neural marker expression, cell number, sterility, mycoplasma. QC check point 4 (final product, before cryopreservation): morphology, neural marker expression, residual undifferentiated hPSCs, cell number, viability, sterility, mycoplasma. Release test (final product, after cryopreservation): neural marker expression, cell number, viability, sterility, mycoplasma, endotoxin, maturation ability test. hPSCs, human pluripotent stem cells; MCB, master cell bank; QC, quality control; WCB, working cell bank
