FIGURE 1.
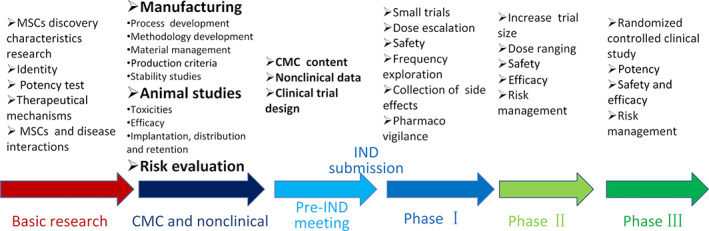
Content and flow chart of mesenchymal stem/stromal cells (MSCs) products development. Process development, formulation selection, process validation, analytical method development and validation, different release and stability specification, nonclinical studies, investigational new drug (IND) application, phase I, phase II, phase III, new drug application (NDA), and commercialization. CMC, chemistry, manufacturing, and controls
