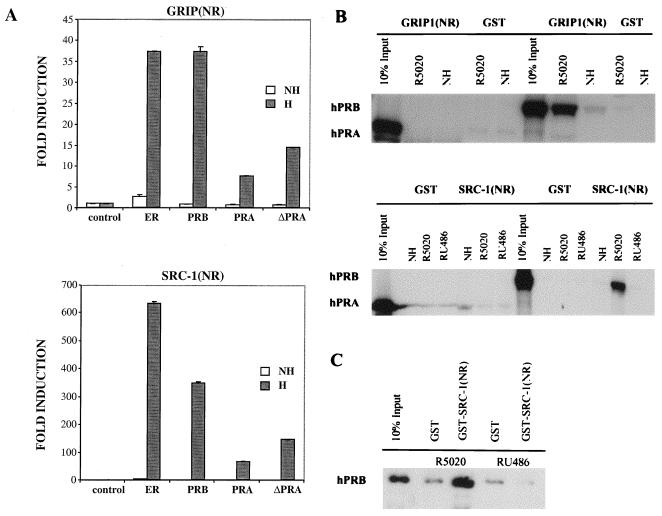
FIG. 10.
hPRA interacts weakly with the NR boxes of the coactivator proteins GRIP1 and SRC-1. (A) HeLa cells were transiently transfected with 0.5 μg of 5XGal4-TATA-LUC, 50 ng of pBKC-βgal, 1 μg of either pM-GRIP1(NR) or pM-SRC-1(NR), 1 μg of either pVP16-T, pVP16-ERα, pVP16-hPRB, pVP16-hPRA, or pVP16-ΔhPRA, and 0.45 μg of pBSII-KS. Transcriptional activity of the luciferase gene was assayed on the 5XGal4-TATA-LUC reporter as in Fig. 2B. Transcriptional activity was measured following the addition of 10−7 M R5020 for PR or 10−7 M 17-β-estradiol for ERα (H); a control was done in the absence of ligands (NH). Transfections were normalized for efficiency as mentioned previously. The data are represented as fold induction over the control interaction between Gal4-GRIP1(NR) or Gal4–SRC-1(NR) and VP16-T for each ligand treatment group, which was normalized to 1.0. Each data point represents the average of triplicate determinations from three separate experiments. The average coefficient of variation at each point was <10% (n = 3). (B and C) GST pull-down assays. The fusion proteins GST-GRIP1(NR) (top) and GST-SRC-1(NR) (bottom) were immobilized on glutathione beads and incubated at 4°C for 24 h with in vitro-translated 35S-hPRA or 35S-hPRB in the presence of vehicle (NH), R5020, or RU486 (B) or baculovirus-purified hPRB bound to R5020 or RU486 (C). The bound baculovirus-purified receptors were analyzed by Western analysis using a polyclonal antibody against PR. An equimolar amount of GST alone was used as a negative control for each condition tested.